

Ames Procedural
Requirements
APR 8715.1
Effective Date: July 16, 2012
Expiration Date: July 16, 2017
COMPLIANCE IS MANDATORY
Ames Health and Safety Procedural Requirements
13.1.1. This chapter of APR 8715.1 establishes minimum requirements to protect laboratory workers from chemical hazards through hazard assessment, hazard communication, training, safe work practices, and special controls for some specific hazardous chemicals.
13.1.2 This chapter of APR 8715.1 together with laboratory safety plans specific to each laboratory together comprise the Center’s written Chemical Hygiene Plan required by 29 CFR 1910.1450, Occupational Exposure to Hazardous Chemicals in Laboratories.
13.1.3 Laboratory Safety Plans:
13.2.1 The Center Director shall designate in writing an employee who is qualified by training or experience to provide technical guidance in the development and implementation of the provisions of the Chemical Hygiene Plan as the installation Chemical Hygiene Officer.
13.2.2 Safety, Health and Medical Services Division shall:
13.2.3 Ames Chemical Hygiene Officer shall:
13.2.4 Supervisors (all levels) shall:
13.2.5 Principal Investigators/Laboratory Chemical Hygiene Officers shall:
13.2.6. Laboratory Employees shall:
13.3.1 Each laboratory must maintain a Laboratory Safety Plan as a site-specific supplement to this Chemical Hygiene Plan.
13.3.2 The Laboratory Safety Plan shall contain laboratory-specific information that will be used to protect employees from health hazards.
13.3.3 It shall include standard operating procedures relevant to health and safety, criteria for determining control measures to reduce employee exposure to hazardous chemicals, instructions for use of personal protective equipment and hygiene practices, measures to ensure proper functioning of fume hoods and other protective equipment, provisions for employee training, designation of circumstances that require prior approval, provisions for medical consultation and medical examinations, and designation of personnel responsible for implementation of the Laboratory safety Plan.
13.3.4. Particular attention shall be given to control measures for operations that involve the use of carcinogens, reproductive hazards, or acutely toxic chemicals.
13.3.5 Laboratory plans shall be reviewed/updated annually, or whenever a new hazard is introduced into the workplace.
13.3.6 Related environmental records, including spill logs and hazardous waste accumulation area inspection logs, are discussed in the Ames Environmental Procedural Requirements APR 8500.1.
13.3.7 Detailed information on the preparation of Laboratory Safety Plans including a suggested outline is provided in Appendix G.
13.4.1 Criteria for Selection of Hazard Control Measures
13.4.1.1 Control measures are required when potential health effects or other operational hazards exist. Control measures, in order of preference, include:
13.4.1.2 OSHA requires a written hazard assessment whenever PPE is used.
13.4.1.2.1 The required PPE hazard assessment process is described in APR 8715.1 Chapter 33 of the Ames Health and Safety Manual.
13.4.1.2.2 The PPE hazard/exposure assessment may be part of the Laboratory Safety Plan.
13.4.1.2.3 Control and protective measures, including provision of PPE and training in both the hazards and appropriate use of PPE, shall be provided before laboratory work begins.
13.4.1.2.4 Factors that shall be considered when determining which control and protective measures to apply include:
13.4.1.2.5 On request, the Ames Chemical Hygiene Officer conducts industrial hygiene review and consultation for operations with hazardous materials.
13.4.2 Administrative Controls and Prior Approval
13.4.2.1 Laboratory management is encouraged to contact the Safety Division for industrial hygiene and occupational safety review prior to the initiation of new hazardous operations. Safety Division personnel may assist in identifying circumstances when there shall be prior approval before implementation of a particular laboratory operation.
13.4.2.2 Projects with radioisotopes, lasers, controlled substances, explosives and propellants, biohazardous materials, nanomaterials, toxic gases, controlled substances, and human subjects have special requirements for review and prior approval.
13.4.2.3 Projects that require use of radioactive materials or equipment that produces ionizing radiation shall be approved in accordance with APR 8715.1 Chapter 7 prior to acquisition of radioactive materials or radiation producing equipment.
13.4.2.4 Projects that require use of lasers or equipment that produces non-ionizing radiation shall be approved in accordance with APR 1700.1 Chapter 8 prior to operation of the laser, UV source, or radiofrequency transmitter.
13.4.2.5 Projects that require use of explosives or propellants shall be approved in accordance with APR 1700.1 Chapter 12 prior to acquisition of explosive materials.
13.4.2.6 Projects that require use of biohazard materials shall be approved by the Biohazard Safety Committee prior to acquisition or use of biohazard Level 2 or biohazard Level 3 materials.
13.4.2.7 Projects that require use of carbon-based nanomaterials shall be approved in accordance with APR 1700.1 Chapter 50 prior to acquisition or production of carbon-based nanomaterials.
13.4.2.8 Principle Investigators who plan to use toxic gases regulated by Santa Clara County shall notify the Occupational Safety, Health and Medical Services Division and the Environmental Management Division prior to acquiring quantities of toxic gases that exceed regulatory thresholds.
13.4.2.9 Principle Investigators who plan to use narcotics or other controlled substances including alcohol in their laboratory research shall follow procedures specified in APR 1700.1 Chapter 23 to acquire, store, use and dispose of those substances.
13.4.2.10 Projects that involve the use of human subjects shall be approved by the Human Subjects Research Review Board prior to commencement of the research activity that involves human subjects.
13.4.2.11 No person shall perform hazardous operations without a responsible supervisor's authorization.
13.4.2.12 All purchase requests for chemicals proceed through line management approval. Purchases by Bank Card shall be subject to the cardholder's written agreement to maintain Hazard Communication and Chemical Hygiene Plan compliance as specified in APR 8715.1 Chapter 24.
13.4.3 General Laboratory Safety Rules
13.4.3.1 General Laboratory Practices: The exposure to hazardous chemicals in the laboratory shall be controlled through the use of good general laboratory practices, standard operating procedures specific to an individual laboratory, engineering controls, and personal protective equipment.
13.4.3.2 The updated National Research Council text, Prudent Practices in the Laboratory, published by the National Academy Press, is adopted as Ames laboratory general procedures (See Appendix D).
13.4.3.3 General Laboratory Safety rules based on recommended Prudent Practices are the primary guidelines for ensuring that laboratory exposure to chemicals is maintained below the permissible limits (see Appendix D for additional information).
13.4.3.4 Four fundamental principles apply to all laboratory operations:
13.4.3.5 Guidelines for working with substances of high toxicity, flammable substances, highly reactive or explosive chemicals, biohazardous materials, and compressed gases are published in references such as the National Research Council publication Prudent Practices in the Laboratory.
13.4.3.6 Specific laboratory practices: Any deviations from general laboratory rules that are necessitated by experiment requirements shall be documented in the Laboratory Safety Plan with an explanation of the circumstances.
13.4.4 Special Hazard Controls
The OSHA Laboratory Standard requires special care for work with known human carcinogens and other substances that are defined as particularly hazardous substances (see Appendix E for additional information).
13.4.4.1 Carcinogens, reproductive toxins or acutely toxic chemicals shall be stored and handled only in a designated area to be specified in the Laboratory Safety Plan. The area can be a single hood, a portion of a room or the entire laboratory.
13.4.4.2 Planned use of carcinogens, reproductive toxins or acutely toxic chemicals shall be evaluated by an industrial hygienist to determine the potential for exposure and the need for containment devices such as fume hoods or gloves boxes.
13.4.4.3 Procedures for safe decontamination or work surfaces and removal of contaminated waste containing carcinogens, reproductive toxins or acutely toxic chemicals shall be documented in the laboratory safety plan.
13.4.4.4 Procedures for working with gases regulated under the Santa Clara County Toxic Gas Ordinance (TGO) shall be based on requirements for regulated gases that are discussed in the Ames Environmental Procedural Requirements, APR 8500.1, EWi-5.3-Toxic Gas Management.
13.4.4.5 All additional provisions for work with particularly hazardous materials shall be incorporated into the standard operation procedures for those materials. Appendix F contains an example SOP.
13.4.5 Chemical Fume Hood Inspection and Maintenance
13.4.5.1 The Safety Division measures ventilation rates and verifies effectiveness of chemical fume hoods annually. Sash location markers and dated calibration stickers are affixed to each fume hood when these checks are performed.
13.4.5.2 Any hood for which air velocity is unacceptable shall be tagged out-of-service until repaired.
13.4.5.3 Fume hoods for radioisotope use meet special requirements described in the Ames Radiation Safety Guide (APR 8715.1, Chapter 7). Note: A current calibration sticker is not a guarantee that the hood is operating properly. For example, if personnel block the exhaust slot openings with equipment, the air velocity shall be affected. If the airflow appears to decrease, the Safety Division may be contacted for assistance.
13.4.6 Personal Protective Equipment
13.4.6.1 Personal protective equipment shall be readily available to laboratory workers for use to reduce exposures to hazardous chemicals in the laboratory.
13.4.6.2 Goggles, gloves, face shields, and aprons are recommended for general laboratory use.
13.4.6.3 Gloves and laboratory aprons shall be worn when any skin contact with carcinogens, reproductive toxins or acutely toxic chemicals is possible.
13.4.6.4 Personal protective equipment shall be provided at no cost to the employee.
13.4.6.5 Specifications for personal protective equipment and criteria for selection of appropriate personal protective equipment are specified in APR 8715.1 Chapter 33, Personal Protective Equipment.
13.5.1 Requirements for Chemical Exposure Assessment
13.5.1.1 Exposure assessment to determine priority for monitoring shall occur when carcinogens, genotoxins, reproductive toxins, and compounds with a high degree of acute toxicity are used regularly (3 or more separate handling sessions per week), used for an extended period of time (greater than 2 to 4 hours at a time), or used in especially large quantities.
13.5.1.2 The exposures of laboratory employees who suspect and report that they have been over-exposed to a toxic chemical in the laboratory, or are displaying symptoms of overexposure to toxic chemicals, will be assessed.
13.5.1.3 The assessment shall initially be qualitative and, based upon the professional judgment of the Ames Chemical Hygiene Officer, may be followed up by specific quantitative monitoring.
13.5.1.4 A memorandum, or report, documenting the assessment shall be sent to the employees involved and their supervisors within fifteen days of receipt of the results.
13.5.1.5 Individual concerns about excessive exposures occurring in the laboratory shall be brought to the attention of the employee’s supervisor or the Laboratory Chemical Hygiene Officer immediately.
13.5.2 Requirements for Exposure Monitoring
13.5.2.1 Exposure in the laboratory to any substance regulated by an OSHA standard that requires monitoring shall be assessed to determine if there is reason to believe that exposure levels for that substance routinely exceed the OSHA action level or exposure limit. Substances regulated by an OSHA standard that requires monitoring include:
13.5.2.2 Routine exposure monitoring of airborne concentrations is not usually warranted in laboratories because:
13.5.3 Occupational Exposure Limits (OEL)
13.5.3.1 ARC shall use OSHA PEL's, Threshold Limit Values (TLV) issued by the American Conference of Governmental Industrial Hygienists (ACGIH) or specific NASA Health Standards issued by the OCHMO, whichever is more stringent, when establishing exposure controls.
13.5.3.2 In the absence of a specific PEL, TLV, or NASA Standard, other sources of occupational exposure limits shall be utilized as specified in NPR 1800.1C.4.2.3.
13.5.3.3 In the absence of a specific PEL, TLV or NASA Standard, the risk assessment process described in Appendix H shall be used.
13.6.1 Each person who handles hazardous materials in the laboratory shall receive task-specific training on spill and accident response.
13.6.2 Spill control and reporting policies, procedures, and requirements are specified in the Ames Environmental Procedural Requirements, APR 8500.1, EWi-5.1 Hazardous Materials Management.
13.6.3 Any employee who may have experienced a hazardous exposure due to a spill, accident, or any other circumstance shall report to the Ames Health Unit for medical consultation.
13.6.4 Information concerning the nature, amount, and circumstances of the exposure shall be provided to the medical professional along with a copy of the MSDS.
13.7.1 The Ames Health Unit provides medical consultation and examination to Government employees with concern about health effects from occupational exposure to chemicals.
13.7.2 A physical examination and/or medical monitoring is provided when physician opinion, employee concerns, regulatory requirements, industrial hygiene survey, or exposure monitoring indicate a need for medical monitoring.
13.7.3 Each employee may review his or her personal medical records upon request.
13.7.4 In an emergency or for the initial evaluation of job-related chemical exposures or accidents, contractors may report to the Ames Health Unit. For routine medical treatment, contractors must report to their own medical clinic.
Training shall be provided:
13.8.1 At the time of initial assignment to a work area where hazardous chemical are present, each employee shall be provided the following information:
13.8.2 Before using hazardous materials, full time laboratory employees shall have completed the following training classes:
13.8.3 Before using hazardous materials, visiting scientists, student interns, and part-time employees shall have completed the following training classes:
13.8.4 Principal Investigator/Laboratory Chemical Hygiene Officers shall provide on-the-job training to employees on:
13.8.5 Supervisors shall verify that laboratory workers have sufficient training to work safely before allowing them to work in a laboratory.
13.8.6 Additional safety and environmental compliance classes related to specific hazards in their work area, such as blood borne pathogens, cryogens, compressed gases, lasers, or radioisotopes are required prior to working with those hazards.
13.8.7 Initial and follow-up training for laboratory workers shall be documented on training records maintained with the Laboratory Safety Plan.
13.8.8 Contractors shall provide/arrange for equivalent training for their employees; they may attend Ames Safety Training classes on a space available basis.
13.9.1 The acquisition of chemicals shall follow the policies and procedures described in the Chemical Hazard Communication Plan, Ames Health and Safety Manual, APR 1700.1, Chapter 24).
13.9.2 Chemical Storage in Laboratories
13.9.2.1 Chemical storage regulations are discussed in detail in the Ames Environmental Procedural Requirements, APR 8500.1.
13.9.2.2 Amounts of chemicals stored in laboratories shall be as small as possible.
13.9.2.3 Storage on bench tops and in hoods is inadvisable.
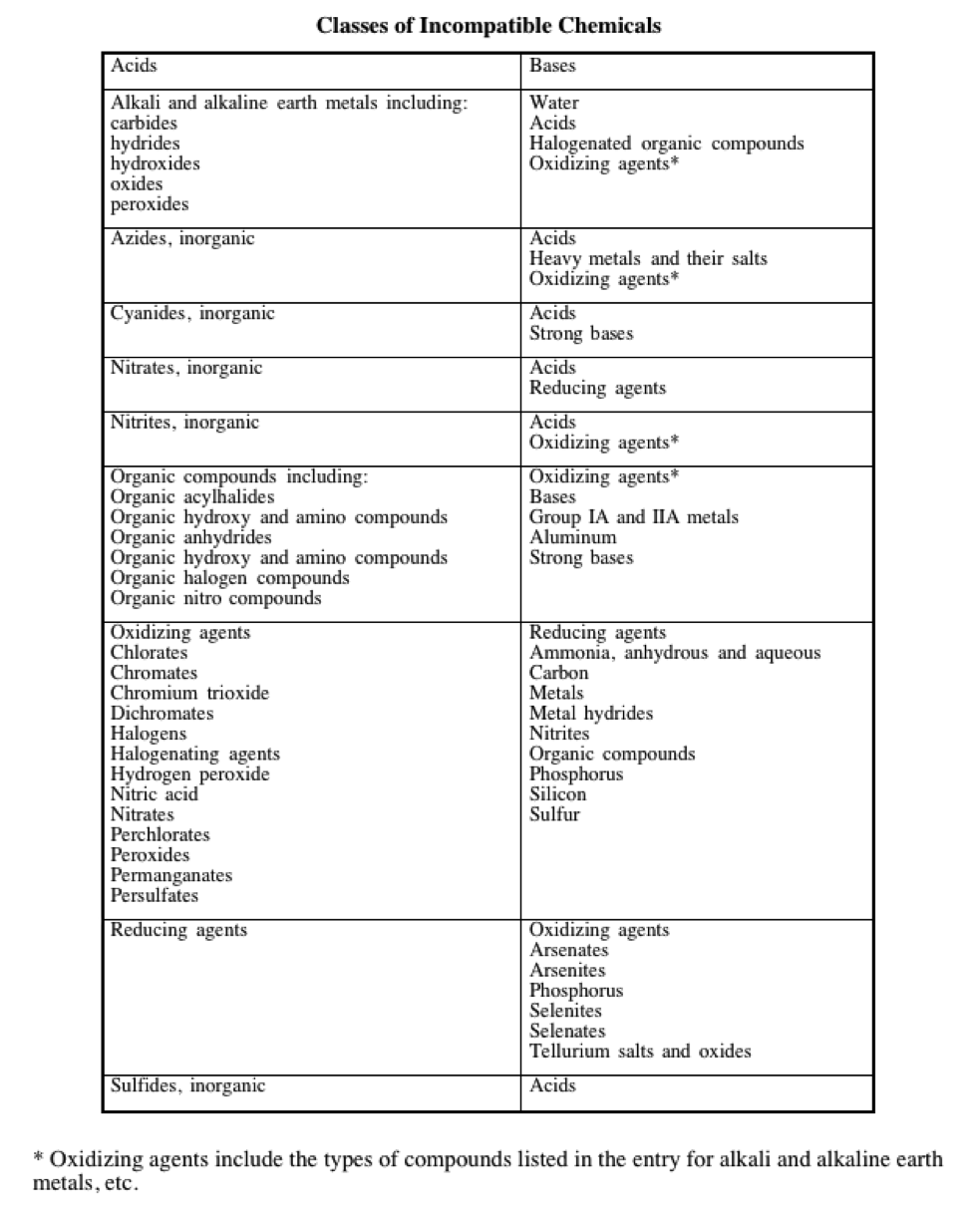
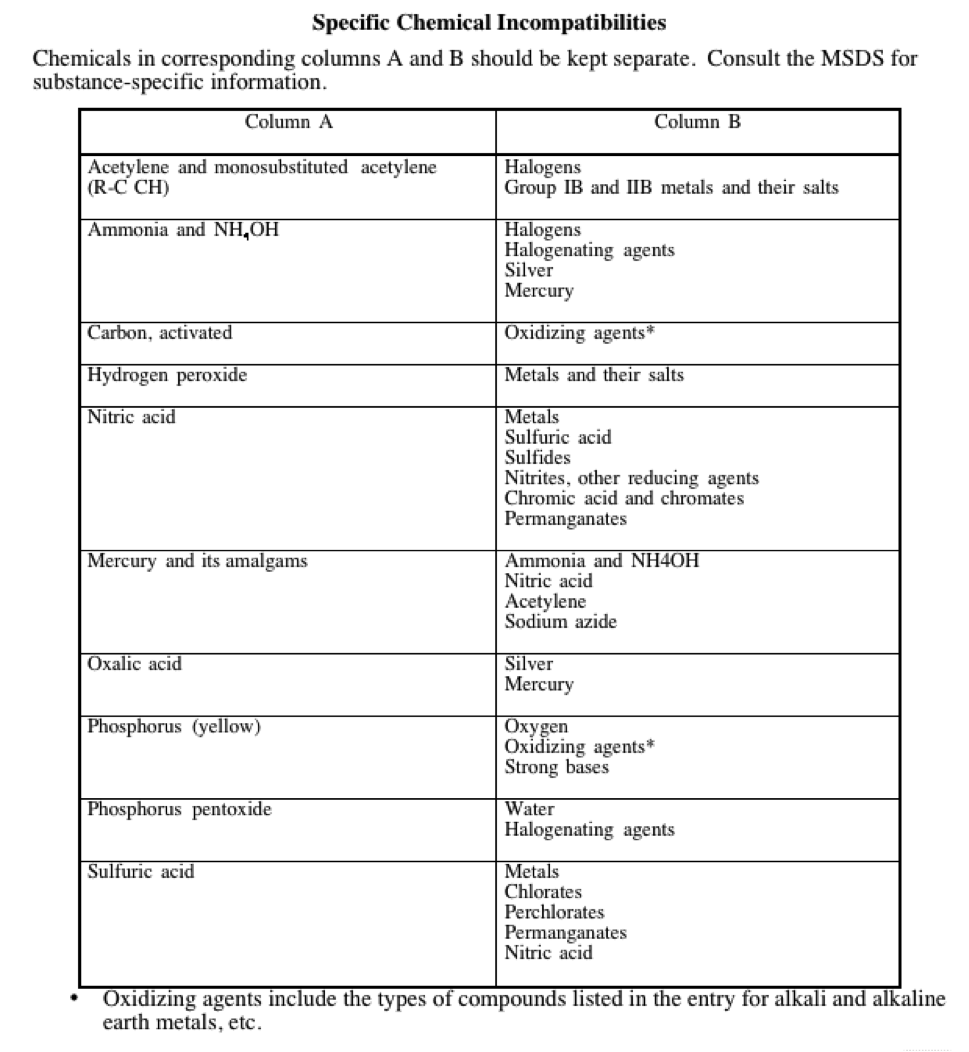
13.9.2.4 Chemicals shall be segregated by hazard class. Non-compatible chemicals shall be separated by location (e.g. separate cabinets) or kept in secondary containment. Chemical compatibility guidelines are referenced in Appendix I.
13.9.3 Picric Acid and Peroxide-forming Chemicals
13.9.3.1 Picric Acid and Peroxide-forming chemicals shall be acquired, labeled, stored, and disposed following guidelines in Appendices J and K.
13.9.3.2 Before acquiring Picric acid, the Lab Safety Plan shall include labeling, testing, and inspection procedures similar to those in Appendix J.
13.9.3.3 Employees shall be trained about the hazards of Picric acid and procedures to use to control these hazards.
13.9.3.4 Before acquiring peroxide-forming chemicals, the Lab Safety Plan shall include labeling, testing, and inspection procedures similar to those in Appendix K.
13.9.3.5 Employees shall be trained about the hazards of peroxides and methods used to control these hazards.
13.9.3.6 Properly dispose of Picric and/or peroxide-forming chemicals according to schedules outlined in Appendices K and L.
13.9.3.7 For ARC policies and procedures on waste accumulation and disposal, refer to Ames Environmental Procedural Requirements, APR 8500.1, EWi-5.1 Hazardous Materials Management And EWi-5.1 Hazardous Waste Management.
13.10.1 Any condition that appears to be an immediate health risk shall be reported to the supervisor, the Safety Division, and 911 immediately.
13.10.2 Hazards shall be reported by any of the methods described in APR 8715.1 Chapter 4.
13.11.1 The Laboratory Safety Plan and its attachments shall be available in each laboratory.
13.11.2 The Ames Health Unit maintains personal medical records, including medical monitoring.
13.11.3 Reports documenting exposure monitoring results shall be provided to involved personnel, the Safety Division and the Ames Health Unit.
13.11.4 The Safety Division maintains exposure-monitoring records and records that document reports of unsafe or unhealthy working conditions.
Operations in chemical laboratories are subject to the procedures and policies listed below when the hazards they address are present in the laboratory:
13.13.1 This Chemical Hygiene Program Plan shall be reviewed annually by the Safety Division.
13.13.2 This document shall be updated by attachments issued as Interim Policy Statements.
13.13.2 Revisions shall incorporate new regulatory requirements and substantially modified procedures initiated since the previous update.
13.13.3 The checklist in Appendix L shall be used to assess implementation of this program.
Action level--an exposure level, calculated as an eight-hour time-weighted average, which initiates certain required activities, such as exposure monitoring, medical surveillance, training and record keeping.
Carcinogen-- A substance that is strongly implicated as a potential cause of cancer in humans and identified by one of the following authorities:
Chemical Hygiene Officer (CHO)--An employee designated to provide technical guidance in the development and implementation of the provisions of the Chemical Hygiene Plan(s). Qualifications include training in industrial hygiene, chemistry, or other pertinent related fields.
Chemical Hygiene Plan (CHP)--A written plan that sets forth procedures, laboratory and control equipment, personal protective gear, and work practices that are capable of protecting employees from the hazards presented by hazardous chemicals used in a particular laboratory workplace.
Chemical Inventory--a written or electronic record of chemicals used in a laboratory, by container, which includes the chemical name of all ingredients, CAS number(s), manufacturer, size of container, owner, and location.
Corrosive Material--Any solid, liquid, or gaseous substance that attacks building materials and metals, and burns, irritates, or destructively attacks organic tissues (mostly notably the skin, lungs, or stomach when taken internally).
Cryogenic Fluids (cryogens)--Elements and compounds that vaporize at temperatures well below room temperature. Most common cryogens have a normal boiling point below approximately 120K. Helium (4.2K), hydrogen (20K), nitrogen (77K), oxygen (90K), and methane (112K) [normal boiling point temperatures in parentheses] are examples of cryogens. Note that the cold vapor of solid carbon dioxide (sublimation temperature of 195 K at one atmosphere) can be considered a cryogenic fluid.
Designated Area--A laboratory, laboratory area, or device such as a fume hood where carcinogens, known human reproductive toxins, or substances with a high degree of acute or chronic toxicity are used. A designated area may be the entire laboratory, an area within the laboratory, or a device such as a laboratory-type hood.
Genotoxin--a toxin that can interact with and alter genetic material. Hazardous Chemical--Any material that, because of its quantity, concentration, or physical or chemical characteristics, poses a significant present or potential hazard to human health and safety or environment if released into the workplace or the environment. If hazardous chemical comprises 1% (0.1% for carcinogens) or greater of a compound or mixture, the compound or mixture must be treated as a hazardous chemical.
Health Hazard--A chemical for which there is statistically significant evidence based on at least one study conducted in accordance with established scientific principles that acute or chronic health effects may occur in exposed employees. Categories of health hazards include:
Laboratory--A facility in which research or analytical chemical procedures are performed, where hazardous materials are stored and used in quantities that may easily be handled by one person (container sizes do not exceed five gallons); a workplace where relatively small quantities of hazardous chemicals are used on a non-production basis.
Laboratory use--means chemical manipulations are carried out on a laboratory scale (containers for reactions, transfers, and other handling can be easily and safely manipulated by one person); multiple chemical procedures or chemicals are used; and procedures involved are not part of a production process, nor do they in any way simulate a production process.
Laboratory Safety Plan (LSP)--contains information about each laboratory operation, including the hazards present, hazards/exposure assessment, hazard control measures, PPE required, identification of carcinogens, genotoxins, reproductive toxins, and compounds with a high degree of acute toxicity used and the location of the designated area for their use, operation-specific training, and employee responsibilities.
Material Safety Data Sheet (MSDS)--Written, printed or electronically transmitted information on the hazards and properties of a particular material, including instructions for its safe use.
Organic Peroxides and Peroxide Formers--Organic peroxides are a special class of compounds with unusual stability problems (stability can be significantly affected by age and contamination). They may be (or become) sensitive to shock, heat, sparks, or other forms of accidental ignition. Some compounds such as ethers auto-oxidize in the presence of oxygen and light to form peroxides.
Permissible Exposure Limit (PEL)--Limit established by OSHA usually expressed as an 8-hour Time Weighted Average (TWA), meaning an airborne contaminant concentration that shall not be exceeded for an 8-hour work shift of a 40 hour work-week. Exposure limits for many hazardous materials are listed in 29 CFR 1910.1000.
Personal Protective Equipment (PPE)--Includes chemical and thermal resistant gloves, safety glasses, goggles and face shields, aprons, respirators, earplugs and muffs, etc.
Physical Hazard--the following categories are included:
Prudent Practices--Recommended guidelines for storage and use of hazardous materials, cited in 29 CFR 1910.1450, based on the National Research Council text Prudent Practices for Handling Hazardous Chemicals in Laboratories, 1981, and the updated text, Prudent Practices in the Laboratory, Handling and Disposal of Chemicals, 1995, National Academy Press, Washington, D.C.
Pyrophoric--A substance that is so rapidly oxidized by oxygen or the moisture in air that it ignites (spontaneous combustion).
Reproductive toxin (known human)--substances that are known to cause adverse effects on the reproductive system, have lethal effects on the fertilized egg developing embryo or fetus, or to cause malformation in the fetus (teratogenesis).
Safety Hazard--Any condition, action, or situation that may result in bodily injury.
Secondary Container--Any chemical container other than an original container that will be used by more than one person or will be used beyond a single workday. This definition should not be confused with secondary containment for chemical release prevention or control.
Short-term exposure limit--a limit usually defined as a 15-minute time-weighted average.
Threshold Limit Value (TLV-TWA)--the exposure limit (established by American Conference of Government Industrial Hygienists), expressed as a time-weighted average airborne "concentration for a normal 8-hour workday and a 40hr work week, to which nearly all workers may be repeatedly exposed, day after day, without adverse effect."
Toxic--Able to injure biological tissue.
Toxicity--The adverse effects resulting from a dose of, or exposure to, a material.
Training--A documented, organized presentation of information fulfilling educational objectives and regulatory requirements.
| ACGIH | American Conference of Governmental Industrial Hygienists |
| ARC | Ames Research Center |
| CAS | Chemical Abstract Number |
| CFR | Code of Federal Regulations |
| CHP | Chemical Hygiene Plan |
| CHO | Chemical Hygiene Officer |
| oC | Degrees Centigrade |
| oF | Degrees Fahrenheit |
| ft2 | Square Feet |
| gal | Gallon |
| HAZCOM | Hazard Communication |
| IARC | International Agency for Research on Cancer |
| K | Kelvin |
| LEL | Lower Explosive Limit |
| MSDS | Material Safety Data Sheet |
| NFPA | National Fire Protection Association |
| NIOSH | National Institute for Occupational Safety and Health |
| NTP | National Toxicology Program |
| OEL | Occupational Exposure Limit |
| OSHA | Occupational Safety and Health Administration |
| PEL | Permissible Exposure Limit |
| PPE | Personal Protective Equipment |
| Ppm | Part(s) per million |
| SOP | Standard Operating Procedure |
| STEL | Short-Term Exposure Limit |
| TGO | Santa Clara County Toxic Gas Ordinance |
| TLV | Threshold Limit Value |
| TWA | Time-Weighted Average |
Directions: This screening tool can be used to document the hazard identification process. The questions are intended to help identify the hazards present or absent in the laboratory. The Laboratory Safety Plan must describe the hazards present and the means of controlling those hazards.
Ionizing and Non-Ionizing Radiation
| Y | N | Does this operation involve... |
|---|---|---|
| Any radiation generating equipment? | ||
| Radioactive materials (including sealed sources and wastes) being generated, processed, used, or stored? | ||
| The use of lasers? | ||
| Any non-ionizing radiation sources NIR? Example of this would be:
|
Chemicals
| Y | N | Are there... |
|---|---|---|
| Any chemicals or toxic materials (including wastes) handled, stored, or generated in this operation? | ||
| Is the chemical inventory current and accurate? | ||
| Is toxicity information (Material Safety Data Sheets can be used) available for all chemicals? | ||
| Is this workplace a laboratory that uses chemicals in "laboratory-scale" operations as described in the Ames Chemical Hygiene Plan (CHP)? |
***If the answer is NO to the above question, then the workplace falls under the hazard communication standard.
Please check the Material Safety Data Sheet or other reference for information on each specific chemical and fill-out the next table.
| Y | N | Are any chemicals handled or generated... |
|---|---|---|
| "selection carcinogens"? | ||
| "carcinogens, genotoxins, reproductive toxins, and compounds with a high degree of acute toxicity"? | ||
| Pyrophoric? | ||
| Peroxide forming chemicals, shock sensitive chemicals or picric acid? | ||
| Toxic or highly toxic gases? | ||
| Flammable or combustible gases, liquids or solids? | ||
| Oxidizer? | ||
| Sensitizer? | ||
| Water-reactive? | ||
| A volatile organic compound? | ||
| Caustic/corrosive? | ||
| Reproductive hazards | ||
| Cryogens? |
| Y | N | Does your operation involve... |
|---|---|---|
| Any tasks where chemicals are mixed that will create an explosive mixture? | ||
| A hazardous exothermic chemical reaction? (example: polymerization) | ||
| A hazardous endothermic chemical reaction? (example: phrolysis) | ||
| Potential for skin absorption of toxic chemical/wastes? (PPE) | ||
| The transportation of chemical over public road? |
Biological
| Y | N | Could a worker be exposed to any biological hazard including the handling of human body fluids or human tissues? |
|---|---|---|
| Is recombinant DNA used? | ||
| Are NIH?CDC regulated agents requiring | ||
| Biosafety level of 2 or greater used? See www.edc.gov for more information | ||
| Is any animal-handling required? See www.cdc.gov for more information |
Physical
| Y | N | Is there any... |
|---|---|---|
| Electrical equipment used in the operation? | ||
| Electrical equipment NOT listed on the Nationally Recognized Testing Laboratories list? | ||
| Electrical equipment built locally involved? | ||
| Work done on or near exposed conductors? | ||
| Potential mechanical energy or mechanical hazards such as motors, pulleys, machinery/shop equipment, forklifts, or hoists and cranes present in this operation? | ||
| Handling, processing, use, or storage (including waste) of explosives? | ||
| Any source of thermal hazards, other than commercially available units that are less that o1C (30F) or greater than 54C (130F)? | ||
| Pressure source (positive or negative) to be considered (i.e. compressed gas cylinders, pressure vessels, hydraulic systems, vacuum systems, etc.) in this operation? | ||
| Are there any sources of excessive noise (e.g., such that you have to shout at a distance of 3 feet to communicate to a coworker or louder than busy traffic) involved in this operation? | ||
| Does this operation include any space that might meet the definition of a confined space? | ||
| *if YES to the above question, will personnel be required to enter the confined spaces? | ||
| Are there any ergonomic issues (repetitive, motion, vibration, lifting.etc.)? | ||
| Does any of the laboratory equipment create a hazard? | ||
| Does this operation involve: the use of equipment, tools or materials outside of the design specifications or outside of the manufacturer's recommendations OR the use of equipment or apparatus built locally? | ||
| Will this operation be left unattended? | ||
| Will operation require work outside normal working hours? | ||
| Will this operation require 2-person rule? | ||
| Will this operation require special attention or contribute to a hazardous condition in the event it is left unexpectedly for long periods of time? |
Environmental
| Y | N | Are there any environmental concerns? If yes, please fill-out the table below. |
|---|---|---|
| Will this operation generate or will personnel be required to handle? | ||
| Hazardous waste? | ||
| Acutely hazardous waste? | ||
| Infectious, medical or biohazardous waste? | ||
| Radioactive waste? | ||
| Mixed waste? | ||
| Air emissions? | ||
| Toxic gas emissions? | ||
| Wastewater discharge? |
Hazard Control Measures
| Y | N | Are measures taken to minimize the potential hazards and mitigate risks? |
|---|---|---|
| Is any local ventilation used in this operation? (Example: chemical hood) |
||
| Is an eyewash/shower available? | ||
| Are interlocks or Lockout/tagout equipment use in the operation? | ||
| Is any personal protective equipment used in this operation? | ||
| Will respiratory protection be required for this operation? | ||
| Have employees received all required training? |
Reprinted with permission from PRUDENT PRACTICES IN THE LABORATORY. Copyright 1995 by the National Academy of Sciences. Courtesy of the National Academy Press, Washington D.C. Qualifying notes for Ames Research Center are printed in italics.
Personal Behavior
Professional standards of personal behavior are required in any laboratory:
Minimizing Exposure to Chemicals
Precautions should be taken to avoid exposure by the principal routes, that is, contact with skin and eyes, inhalation, and ingestion.
1. Avoiding Eye Injury
Ordinary prescription glasses do not provide adequate protection against injury. Prescription safety glasses and goggles can be obtained.
Contact lenses offer no protection against eye injury and cannot be substituted for safety glasses and goggles. It is best not to wear contact lenses when carrying out operations where chemical vapors are present or a chemical splash to the eyes or chemical dust is possible because contact lenses can increase the degree of harm and can interfere with first aid and eye-flushing procedures. If an individual must wear contact lenses for medical reasons, then safety glasses with side shields or tight-fitting safety goggles must be worn over the contact lenses. Employees are advised to inform the supervisor when contact lenses will be worn for operations with hazardous chemicals.
2. Avoiding Ingestion of Hazardous Chemicals
Eating, drinking, smoking, gum chewing, applying cosmetics, and taking medicine in laboratories where hazardous chemicals are used should be strictly prohibited. Food, beverages, cups, and other drinking and eating utensils should not be stored in areas where hazardous chemicals are handled or stored. Glassware used for laboratory operations should never be used to prepare or consume food or beverages. Laboratory refrigerators, ice chests, cold rooms, ovens, and so forth should not be used for food storage or preparation. Laboratory water sources and de-ionized laboratory water should not be used for drinking water.
Laboratory chemicals should never be tasted. A pipette bulb or aspirator should be used to pipette chemicals or to start a siphon; pipetting should never be done by mouth. Hands should be washed with soap and water immediately after working with any laboratory chemicals, even if gloves have been worn.
3. Avoiding Inhalation of Hazardous Chemicals
Toxic chemicals or compounds of unknown toxicity should never be smelled. Procedures involving volatile toxic substances and operations involving solid or liquid toxic substances that may result in the generation of aerosols should be conducted in a laboratory hood. Dusts should be recognized as potentially contaminated and hazardous. Hoods should not be used for disposal of hazardous volatile materials by evaporation. Such materials should be treated as chemical waste and disposed of in appropriate containers in accord with institutional procedures.
The following general rules should be followed when using laboratory hoods:
Note: For more information, see OSHA Personal Protective Equipment Standard (29 CFR 1910.132-138) for hand protection.
6. Clothing and Protective Apparel
Long hair and loose clothing or jewelry must be confined when working in the laboratory. Unrestrained long hair, loose or torn clothing, and jewelry can dip into chemicals or become ensnared in equipment and moving machinery. Clothing and hair can catch fire. Sandals and open-toed shoes should never be worn in a laboratory in which hazardous chemicals are in use.
It is advisable to wear a laboratory coat when working with hazardous chemicals. This is particularly important if personal clothing leaves skin exposed. Apparel giving additional protection (e.g., non-permeable laboratory aprons) is required for work with certain hazardous substances. Because many synthetic fabrics are flammable and can adhere to the skin, they can increase the severity of a burn. Therefore, cotton is the preferred fabric.
Housekeeping
There is a definite correlation between orderliness and level of safety in the laboratory. In addition, a disorderly laboratory can hinder or endanger emergency response personnel. The following housekeeping rules should be adhered to:
Transport of Chemicals
Chemicals being transported outside the laboratory or between stockrooms and laboratories should be in break-resistant secondary containers. Secondary containers commercially available are made of rubber, metal, or plastic, with carrying handle(s), and are large enough to hold the contents of the chemical containers in the event of breakage. When transporting cylinders of compressed gases, the cylinder should always be strapped in a cylinder cart and the valve protected with a cover cap. When cylinders must be transported between floors, passengers should not be in the elevator.
Storage of Chemicals
The accumulation of excess chemicals can be avoided by purchasing the minimum quantities necessary for a research project. All containers of chemicals should be labeled properly. Any special hazards should be indicated on the label. For certain classes of compounds (e.g., ethers and other as peroxide formers), the date the container was opened should be written on the label. Peroxide formers should have the test history and date of discard written on the label as well. Only small quantities (less than 1 liter (L)) of flammable liquids should be kept at workbenches. Larger quantities should be stored in approved storage cabinets. Quantities greater than 1 L should be stored in metal or break-resistant containers. Large containers (more than 1 L) should be stored below eye level on low shelves. Hazardous chemicals and waste should not be stored on the floor.
Refrigerators used for storage of flammable chemicals must be explosion-proof, laboratory-safe units. Materials placed in refrigerators should be clearly labeled with water-resistant labels. Storage trays or secondary containers should be used to minimize the distribution of material in the event a container should leak or break. It is good practice to retain the shipping can for such secondary containers.
All chemicals should be stored with attention to incompatibilities so that if containers break in an accident, reactive materials do not mix and react violently.
Disposal of Chemicals
Note: This section contains practical guidelines for waste accumulation. For ARC policies and procedures, refer to Ames Environmental Procedural Requirement, APR 8500.1, EWi -5.2 Hazardous Waste Management.
Virtually every laboratory experiment generates some waste, which may include such items as used disposable lab-ware, filter media and similar materials, aqueous solutions, and hazardous chemicals. The overriding principle governing the handling of waste in prudent laboratory practice is that no activity should begin unless a plan for the disposal of nonhazardous and hazardous waste has been formulated. Application of this simple rule will ensure that the considerable regulatory requirements for waste handling are met and that unexpected difficulties, such as the generation of a form of waste (e.g., chemical-radioactive-biological) that the institution is not prepared to deal with, are avoided.
Each category of waste has certain appropriate disposal methods. In choosing among these methods, several general principles apply, but local considerations can strongly influence the application of these rules:
Note: The Safety Office will provide consultation and guidance on request.
Note: Approved containers are provided on request by the Ames contractor for hazardous waste.
Note: The Ames Chemical Exchange (ACE) program for sharing excess chemicals is described in the Ames Environmental Procedural Requirements.
Note: Ames policy is described in the Ames Procedural Requirement, APR 8500.1, EWi -5.2 Hazardous Waste Management.
Use and Maintenance of Equipment and Glassware
Good equipment maintenance is essential for safe and efficient operations. Laboratory equipment should be inspected and maintained regularly and serviced on schedules that are based on both the likelihood of and the hazards from failure. Maintenance plans should ensure that any lockout procedures cannot be violated.
Careful handling and storage procedures should be used to avoid damaging glassware. Chipped or cracked items should be discarded or repaired. Vacuum-jacketed glassware should be handled with extreme care to prevent implosions. Evacuated equipment such as Dewar flasks or vacuum desiccators should be taped or shielded. Only glassware designed for vacuum work should be used for that purpose.
Hand protection should be used when picking up broken glass. Small pieces should be swept up with a brush into a dustpan. Glassblowing operations should not be attempted unless proper annealing facilities are available. Adequate hand protection should be used when inserting glass tubing into rubber stoppers or corks or when placing rubber tubing on glass hose connections. Cuts from forcing glass tubing into stoppers or plastic tubing are the most common kind of laboratory accident and are often serious. Tubing should be fire polished or rounded and lubricated, and hands should be protected with toweling and held close together to limit movement of glass should it fracture. The use of plastic or metal connectors should be considered.
Handling Flammable Substances
Flammable substances present one of the most widespread hazards encountered in the laboratory. Because flammable materials are employed in so many common laboratory operations, basic prudent laboratory practice should always assume the presence of fire hazard unless a review of the materials and operations in the laboratory verifies the absence of significant hazard. For example, simple operations with aqueous solutions in a laboratory where no flammable organic liquids are present involve no appreciable fire hazard. In all other circumstances, the risk of fire should be recognized and kept to a minimum.
For a fire to start, an ignition source, fuel, and oxidizer must be present. Prudent laboratory practice in avoiding fire is based on avoiding the presence of one of these components. The flammability and explosive characteristics of the materials being used should be known. Solvent labels, LCSSs, or other sources of information can be consulted to learn the flash point, vapor pressure and explosive limit in air of each chemical handled. While all flammable substances should be handled prudently, the extreme flammability of some materials requires additional precautions.
To ensure that laboratory workers respond appropriately, they should be briefed on the necessary steps to take in case of a fire. The laboratory should be set up in such a way that the locations of fire alarms, pull stations, fire extinguishers, safety showers, and other emergency equipment are marked and all laboratory personnel alerted to them. Exit routes in case of fire should be reviewed. Fire extinguishers in the immediate vicinity of an experiment should be appropriate to the particular fire hazards. Proper extinguishers must be used because fires can be exacerbated by use of an inappropriate extinguisher. Telephone numbers to call in case of an accident should be readily available.
Responsibility for Unattended Experiments and Working Alone
Generally, it is prudent to avoid working alone at the bench in a laboratory building. Individuals working in separate laboratories outside of working hours should make arrangements to check on each other periodically, or ask security guards to check on them. Experiments known to be hazardous should not be undertaken by a worker who is alone in a laboratory. Under unusually hazardous conditions, special rules may be necessary.
Laboratory operations involving hazardous substances are sometimes carried out continuously or overnight with no one present. It is the responsibility of the worker to design these experiments so as to prevent the release of hazardous substances in the event of interruptions in utility services such as electricity, cooling water, and inert gas. Laboratory lights should be left on, and signs should be posted identifying the nature of the experiment and the hazardous substances in use. If appropriate, arrangements should be made for other workers to periodically inspect the operation. Information should be posted indicating how to contact the responsible individual in the event of an emergency.
PRUDENT PRACTICES IN THE LABORATORY. Copyright 1995 by the National Academy of Sciences. Chapter 5D
Note: Guidelines have been adapted for Ames Research Center policies and procedures.
Approvals
Prepare a Standard Operating Procedure for use of these materials and obtain the approval of the laboratory supervisor. Attach the SOP to the Laboratory Safety Plan. See suggested format (Appendix G).
Access
Conduct all transfers and work with these substances in a "designated area;" (hood, glove box, or portion of a lab). Document the location and that all people with access are aware of the substances being used and necessary precautions in the SOP.
Fume Hoods
Always use a hood (previously evaluated to confirm adequate performance with an average face velocity of 100+ 20 linear feet per minute (ARC standard) or other containment device for procedures that may result in the generation of aerosols or vapors containing the substance.
Glove Boxes
Contact the Safety Division for assistance.
Decontamination
Document decontamination procedures in the SOP. Decontamination vacuum pumps or other contaminated equipment, including glassware, in the hood before removing them from the designated area. Decontaminate the designated controlled area before normal work is resumed there.
Exiting
On leaving a controlled area, remove any protective apparel (placing it in an appropriate, labeled container) and thoroughly wash hands, forearms, face, and neck.
Medical surveillance
If using toxicologically significant quantities of such a substance on a regular basis (e.g., 3 times per week), consult with the Ames Chemical Hygiene Officer concerning desirability of regular medical surveillance. Include this information in the SOP.
Records
Keep accurate records of the amounts of Particularly Hazardous Chemicals stored and used, the dates of use, and names of users.
Signs and labels
Assure that the designated area is conspicuously marked with warning and restricted access signs.
Assure that all storage cabinets and containers of these substances are appropriately labeled with identity and warning labels.
Storage
Store containers of Particularly Hazardous Chemicals only in a ventilated, limited access area in appropriately labeled, unbreakable, chemically resistant containers.
Spills
Assure that contingency plans, equipment, and materials to minimize exposures of people and property in case of accident are available. (See Ames Environmental Procedural Requirements, APR 8500.1, EWi 5.1 Hazardous Materials Management;) for spill reporting requirements)
Waste
Ensure that containers of contaminated waste (including washings from contaminated flasks) are transferred from the designated area in a secondary container under the supervision of authorized personnel.
Bag, label, and dispose as hazardous waste any contaminated clothing or shoes.
Store contaminated waste in closed, suitably labeled, impervious containers.
See also: APR 8500.1, EWi 5.2, Hazardous Waste Management

The Laboratory Safety Plan must contain laboratory-specific information that will be used to protect employees from health hazards. It must include standard operating procedures relevant to health and safety, criteria for determining control measures to reduce employee exposure to hazardous chemicals, instructions for use of personal protective equipment and hygiene practices, measures to ensure proper functioning of fume hoods and other protective equipment, provisions for employee training, designation of circumstances that require prior approval, provisions for medical consultation and medical examinations, and designation of personnel responsible for implementation of the Laboratory safety Plan.
The Laboratory Safety Plan may also include information about the facility and evaluation of potentially hazardous operations (The Laboratory Safety Plan can be used to document both the supervisor’s hazard assessment and the PPE assessment required by OSHA). Standard Operating Procedures that contain safety instructions and precautions for hazardous operation may be included in the laboratory safety plan or their location referenced.
The Ames Chemical Hygiene Officer can assist researchers in developing safety procedures for specific hazards. Laboratory plans are reviewed/updated annually, or whenever a new hazard is introduced into the workplace. Related environmental records, including spill logs and hazardous waste accumulation area inspection logs, are discussed in the Ames Environmental Procedural Requirements APR 8500.1.
Suggested Outline
Evaluate the risk to the employee from the hazards present and describe the control measures. See Appendix H: Laboratory Risk Assessment, for additional information.
Example:
| Hazard/Exposure | Hazard Scenario | Risk | Control Measures |
|---|---|---|---|
| Describe the major hazards/exposure present | Describe how the employee could be exposed to the hazard | Discuss the risks based upon the hazard and the hazard scenario | Describe how the employee will be protected from the hazard and the risk of injury/illness minimized (i.e., applicable exposure limits, engineering controls to be used, PPE to be worn, training to be provided, etc) |
— Laboratory-specific rules, procedures, and controls (See Appendix C).
These are based upon the hazards/exposures identified.
Examples:
— Standard Operating Procedures for work with "Particularly Hazardous Substances" or other high hazard operations (see Appendices E and F for additional information):
Laboratories deal with substances known to be hazardous, with substances with unknown hazard potential, and with unique/innovative experiments. Laboratory workers usually handle only small amounts of materials, laboratory exposures are typically brief and infrequent, and the need to use particular substances is often difficult to predict in advance. This appendix provides guidance for assessing the hazards in the laboratory.
For each chemical consider the toxicity, frequency and duration of use, the quantity of material handled, and the inhalation and skin exposure potential. Reference: PRUDENT PRACTICES IN THE LABORATORY. Copyright 1995 by the National Academy of Sciences. Courtesy of the National Academy Press, Washington, D.C. Quick Guide to Risk Assessment for Hazardous Chemicals
The major items:
Note the signs and symptoms of exposure to the chemicals to be used in the proposed experiment. Note appropriate measures to be taken in the event of exposure or accidental release of any of the chemicals.
Toxicity:
Review the Material Safety Data Sheets and other available reference for toxicology and health hazard information. The table below can be used to assist the rating of acutely toxic chemicals.
Low: transitory, mild health effects
Moderate: effects from which the person would recover; would not produce chronic effects that would negatively impact on the quality of life.
High: all compounds which meet the "Particularly Hazardous Chemical" definition and any compound which has no available toxicity information.
| Hazard Level | Toxicity Rating | Oral LD50 (Rats, per kg) | Skin Contact LD50 (Rabbits, per kg) | Inhalation LC50 (Rats, ppm for 1h) | Inhalation LC50 (Rats, mg/m3 for 1h) |
|---|---|---|---|---|---|
| High | Highly toxic | <50 mg | <200 mg | <200 | <2,000 |
| Medium | Moderately toxic | 50 to 500 mg | 200 mg to 1 g | 200 to 2,000 | 2,000 to 20,000 |
| Low | Slightly toxic | 500 mg to 5 g | 1 to 5 g | 2,000 to 20,000 | 20,000 to 200,000 |
Reprinted with permission from PRUDENT PRACTICES IN THE LABORATORY. Copyright 1995 by the National Academy of Sciences. Courtesy of the National Academy Press, Washington, D. C. Table 3.1.
Routes of Exposure:
Inhalation and skin exposure are the most probable types of exposure for laboratory workers. Accidental injection can also occur if needles or other sharp implements are used in any procedures. The actual potential for an exposure to occur can be reduced by the use of hazard/exposure control measures.
Examples:
Inhalation Exposure Potential:
Skin Exposure Potential: Evaluate this exposure route independently of the inhalation route. Consider (1) the potential for contact with unprotected skin and the potential for surface contamination (e.g. dusts); (2) frequency of use; and, (3) the chemical's ability to be absorbed through the skin.
If the risk/exposure potential is high or moderate, the experimental protocol should be adjusted, whenever possible, to reduce the level. The correct use of engineering controls and personal protection equipment lowers the exposure potential. If the assessment is still high, contact the Ames Chemical Hygiene Officer for further evaluation.
Reprinted (adapted) with permission from Prudent Practices in the Laboratory. Copyright 1995 by the National Academy of Sciences. Courtesy of the National Academy Press, Washington, D.C.
Chemicals in the two columns should be kept separate. Consult the MSDS for substance-specific information.
Picric Acid and other multi-nitro aromatic compounds form shock-sensitive explosive crystals when dry. The Department of Transportation (DOT) classifies Picric Acid as a Class 1.1D Explosive if it contains less than 30% water by mass. If the material contains greater than 30% moisture, DOT classifies it as a Class 4.1 Flammable Solid. It is even more dangerous when stored in a container with a metal cap, as it can then form metal picrate salts, which are extremely shock-sensitive.
Guidelines for Storage of Picric Acid and other Multi-Nitro Aromatic Compounds: Principal Investigators/Chemical Hygiene Officers are responsible for identifying picric acid and other hazardous multi-nitro aromatics used in the work area. These PI's/Chemical Hygiene Officers are also responsible for ensuring that the following occur:
If relocating from the lab, take material with you or ensure that next occupant is aware of presence of and dangers of the material, its date of purchase, its hydration status. Coordinate transfers and relocations with Code JQ
Many oxygenated organic compounds can spontaneously form explosive peroxides with age, via a free-radical auto-oxidation reaction. Although exposure to light and air enhances the formation of peroxides, there are examples of peroxides forming in unopened, shielded containers.
Unfortunately, though there are various tests for detecting peroxide-forming chemicals, there are no definitive data available regarding the specific conditions under which these peroxides will reach explosive levels. Several common test methods, such as using dip test strips, may detect some but not all types of unstable peroxides (e.g. some polyperoxides are not detected), and deperoxidation procedures might not mitigate all the hazards present.
In addition to the problems with testing for peroxides, with deperoxidation, and with defining the conditions that make peroxide-forming chemicals hazardous, there are no specific federal or Cal/OSHA regulations that address the subject matter. Despite this uncertainty, NASA-ARC has developed the following guidelines for employees to follow in handling peroxide forming chemicals. Guidelines for Storage/Testing/Disposal of Peroxide Forming Chemicals: Principal Investigators/Chemical Hygiene Officers are responsible for identifying peroxide forming chemicals used in the work area. They are also responsible for ensuring that the following occur:
All the above test strips are the gentlest, fastest, easiest, and the most accurate of all testing methods (5,7). However, although these strips can detect hydroperoxides and most higher peroxides, some polyperoxides are not detected . Therefore, it is recommended that no distillation shall occur if the levels of peroxides detected are above 25 ppm (9).
Label for Peroxide Forming Chemicals:
Warning: May form Explosive Peroxides
Store, handle, and dispose of as per Ames' Peroxide Forming Chemical Policy, as outlined in Chemical Hygiene Program (Chapter 13 of Ames Health and Safety Manual. Keep in tightly closed, original container. Avoid exposure to light, air, and heat.
If crystals, discoloration, or layering are visible, do not open! Contact EH&S Hazardous Waste immediately for guidance 4-5360. Check for peroxides before distilling. Do not distill if peroxide concentration is > 25 ppm. If concentration is > 100 ppm, do not touch material! Call Hazardous Waste at x4-5360 immediately for disposal.
This chemical has a limited shelf life. Dispose of prior to expiration date:
Date Received:_________ Expiration (Disposal) Date*:________
*(as per "Disposal Guidelines for Peroxidizable Chemicals, Table 1, Appendix I, "Peroxide Forming Chemicals," Chapter 13, Ames Health and Safety Procedural Requirements, APR 8715.1)
Training:
Employees who either handle or who may be exposed to hazardous materials, including peroxide-forming chemicals, must complete the course, "Chemical Hygiene for Laboratories," Course No. A136. All employees in the work area require on-the-job training on the specific hazards and controls of the materials being handled. Area specific training is a line management responsibility.
Engineering Controls:
| Table 1: Disposal Guidelines for Peroxidizable Chemicals* | |
|---|---|
| Peroxidizable Chemical Classification | Dispose After |
| List A Chemicals | 3 months |
| List B Chemicals | 12 months |
| List C, uninhibited chemicals | 24 hours |
| List C, inhibited chemicals | 12 months |
| List D Chemicals | 12 months |
*Modified, from: National Research Council, Prudent Practices in the Laboratory, Handling and Disposal of Chemicals; National Academy Press; Washington, D.C., 1999, and Kelly, Richard, "Review of Safety Guidelines for Peroxidizable Organic Chemicals," Chemical Health And Safety, American Chemical Society, Sept/Oct 1996, pp. 28-36
The following lists of chemicals are not exhaustive. Principal Investigators must consult the MSDSs and other information sources for the chemicals used in their work areas to determine the potential for peroxide-formation.
| List A- Chemicals that form explosive levels of peroxides without concentration (Safe storage limit - 3 months) |
||||
|---|---|---|---|---|
| Chemical | CAS | Synonyms | State | Reference |
| Butadiene(1) | 106-99-0 | 1,3-Butadiene | l | 4 |
| Chloroprene (1) | 126-99-8 | 2-Chloro-1,3- butadiene | l | 4 |
| Divinyl acetylene | 821-08-9 | 1,5-Hexadien- 3-yne | l | 5 |
| Isopropyl ether | 108-20-3 | l | 5 | |
| Tetrafluoroethylene (1) | 116-14-3 | l | 4 | |
| Vinyl ether | 109-93-3 | Divinyl ether | l | 5 |
| Vinylidene chloride | 75-35-4 | 1,1- Dichloroethylene | l | 5 |
| List B-Chemicals that form explosive levels of peroxides on concentration (Safe storage time-12 months) | ||||
|---|---|---|---|---|
| Chemical | CAS | Synonyms | State | Reference |
| Acetal | 105-57-7 | l | 5 | |
| Acetaldehyde | 75-07-0 | l | 4 | |
| Benzyl alcohol | 100-51-6 | l | 4 | |
| 2-Butanol** | 78-92-2 | l | 4 | |
| Cyclohexanol | 108-93-0 | l | 4 | |
| Cyclohexene | 110-83-8 | l | 5 | |
| 2-Cyclohexen-1-ol | 822-67-3 | l | 4 | |
| Cyclopentene | 142-29-0 | l | 5 | |
| Decahydronaphthalene | 91-17-8 | l | 4 | |
| Diacetylene | 460-12-8 | g | 5 | |
| Dicyclopentadiene | 77-73-6 | l | 5 | |
| Diethylene glycol dimethyl ether | 111-96-6 | Diglyme | l | 5 |
| Dioxane | 123-91-1 | 1,4-Dioxane | l | 5 |
| Ethylene glycol dimethyl ether | 110-71-4 | Glyme | l | 5 |
| Ethyl ether | 60-29-7 | Diethyl ether | l | 5 |
| Furan | 128-37-0 | l | 5 | |
| 4-Heptanol | 589-55-9 | l | 4 | |
| 2-Hexanol** | 626-93-7 | l | 4 | |
| Isopropyl benzene | 98-82-8 | Cumene | l | 5 |
| Methyl acetylene | 74-99-7 | Propyne | g | 5 |
| 3-Methyl-1-butanol | 123-51-3 | Isoamyl alcohol | l | 4 |
| Methyl cyclopentane | 96-37-7 | l | 5 | |
| Methyl isobutyl ketone | 108-10-1 | Methyl-i-butyl ketone | l | 5 |
| 4-Methyl-2-pentanol | 108-11-2 | l | 4 | |
| 2-Pentanol** | 6032-29-7 | l | 4 | |
| 4-Penten-1-ol | 821-09-0 | l | 4 | |
| 1-Phenylethanol | 98-85-1 | alpha-Methyl-benzyl alcohol | l | 4 |
| 2-Phenylethanol | 60-12-8 | Phenethyl alcohol | l | 4 |
| Tetrahydrofuran | 109-99-9 | l | 5 | |
| Tetrahydronaphthalene | 119-64-2 | l | 5 | |
| List C- Chemicals which may autopolymerize as a result of peroxide accumulation (Safe storage time: inhibited chemicals- 12 months; uninhibited chemicals: - 24 hours) |
||||
|---|---|---|---|---|
| Chemical | CAS | Synonyms | State | Reference |
| Acrylic acid(2) | 79-10-7 | l | 5 | |
| Acrylonitrile(2) | 107-13-1 | l | 5 | |
| Butadiene(3) | 106-99-0 | g | 5 | |
| Buten-3-yne | 689-97-4 | Vinyl acetylene & Butenyne | g | 5 |
| Chloroprene(3) | 126-99-8 | 2-Chloro-1,3-butadiene | g | 5 |
| Chlorotrifluoroethylene | 79-38-9 | g | 5 | |
| Methyl methracrylate(2) | 80-62-6 | l | 5 | |
| Styrene | 100-42-5 | l | 5 | |
| Tetrafluoroethylene(3) | 116-14-3 | g | 5 | |
| Vinyl acetate | 108-05-4 | l | 5 | |
| Vinyl chloride | 75-01-4 | Mono-chloroethylene | g | 5 |
| Vinylidene chloride | 75-35-4 | 1,1-Dichloroethylene | l | >5 |
| 2-Vinyl pyridine | 100-69-6 | l | 5 | |
| 4-Vinyl pyridine | 100-43-6 | l | 5 | |
Notes: ** Please note that secondary alcohols can be peroxide-forming chemical hazards. However, this is true only for the anhydrous form of the alcohols, and it is also only true if they are used in chemical processes (e.g., heating, distilling, performing chemical reactions, performing bulk evaporations). If these chemicals are used only for wipe cleaning or solvent extractions, there is no evidence that these secondary alcohols are dangerous.
| List D: Chemicals that may form peroxides but cannot be clearly placed in Lists A-C. | |||
|---|---|---|---|
| Acrolein | 107-02-8 | Isobutyl vinyl ether | 109-53-5 |
| Allyl ether | 557-40-4 | Isophrone | 78-59-1 |
| Allyl ethyl ether | 537-31-3 | b-Isoproxypropionitile | |
| Allyl phenyl ether | 1746-13-0 | tert-Butyl methyl ether | 16634-04-0 |
| p-(n-Amyloxy)benzoyl chloride | 36823-84-4 | n-Butyl phenyl ether | 1126-79-0 |
| n-Amyl ether | 693-65-2 | n-Butyl vinyl ether | 11-34-2 |
| Benzyl n-butyl ether | 3459-80-1 | Chloroacetaldehyde diethylacetal | 621-62-5 |
| Benzyl ether | 103-50-4 | 2-Chlorobutadiene | 126-99-8 |
| Benzyl ethyl ether | 539-30-01 | 1-(2-Chloroethoxy)-2-phenoxyethane | 2243-49-91 |
| Benzyl methyl ether | 558-86-3 | Chloroethylene | 75-01-4 |
| Benzyl-1-napthyl ether | 613-62-7 | Chloromethyl methyl ether | 107-30-2 |
| 1,2-Bis(2-chloroethoxyl)ethane | 112-26-5 | b-Chlorophenetole | 614-72-2 |
| Bis(2-ethoxyethyl)ether | 112-36-7 | o-Chorophenetole | |
| Bis(2-(methoxyethoxy)ethyl) ether | 143-24-8 | Cyclooctene | 931-88-4 |
| Bis(2-chloroethyl) ether | 111-44-4 | Cyclopropyl methyl ether | 540-47-6 |
| Bis(2-ethoxyethyl) adipate | 109-44-4 | Diallyl ether | 557-40-4 |
| Bis(2-methoxyethyl) carbonate | p-Di-n-butoxybenzene | 75942-37-9 | |
| Bis(2-methoxyethyl) ether | 119-96-6 | 1,2-Dibenzyloxyethane | |
| Bis(2-methoxyethyl) phthalate | 117-82-8 | p-Dibenzyloxybenzene | |
| Bis(2-methoxymethyl) adipate | 106-06-3 | 1,2-Dichloroethyl ethyl ether | 623-46-1 |
| Bis(2-n-butoxyethyl) phthalate | 117-83-9 | 2,4-Dichlorophenetole | 5392-86-9 |
| Bis(2-phenoxyethyl) ether | 622-87-7 | Diethoxymethane | 462-95-3 |
| Bis(4-chlorobutyl) ether | 6334-96-9 | 2,2-Diethoxypropane | 126-84-1 |
| Bis(chloromethyl) ether | 542-88-1 | Diethyl ethoxymethylenemalonate | 87-13-8 |
| 2-Bromomethyl ethyl ether | 13057-17-5 | Diethyl fumarate | 623-91-6 |
| beta-Bromophenetole | 596-10-6 | Diethyl acetal | 105-57-7 |
| o-Bromophenetole | 593-19-7 | Diethylketone | 96-22-0 |
| p-Bromophenetole | m,o,p-Diethoxybenzene | 2168-54-9 | |
| 3-Bromopropyl phenyl ether | 588-63-6 | 1,2-Diethoxyethane | 629-14-1 |
| Di(1-propynl) ether | 111-43-4 | Dimethoxymethane | 109-87-5 |
| Di(2-propynl) ether | 1,1-Dimethoxyethane | 534-15-6 | |
| Di-n-propoxymethane | 505-84-0 | n-Methylphenetole | |
| 1,2-Epoxy-3-isopropoxypropane | 2-Methyltetrahydrofuran | 202-507-4 | |
| 1,2-Epoxy-3-phenoxypropane | 122-60-1 | 3-Methoxy-1-butyl acetate | 4435-53-4 |
| p-Ethoxyacetophenone | 11676-63-7 | 2-Methoxyethanol | 109-86-4 |
| 1-(2-Ethoxyethoxy)ethyl acetate | 3-Methoxyethyl acetate | 110-49-6 | |
| 2-Ethoxyethyl acetate | 111-15-9 | 2-Methoxyethyl vinyl ether | 111-96-6 |
| (2-Ethoxyethyl)-a-benzoyl benzoate | Methoxy-1,3,5,7-cyclooctatetraene | ||
| 1-Ethoxynaphthalene | 5328-01-8 | b-Methoxypropionitrile | 110-67-8 |
| o,p-Ethoxyphenyl isocyanate | 5395-71-1 | m-Nitrophenetole | |
| 1-Ethoxy-2-propyne | 1-Octene | 203-893-7 | |
| 3-Ethoxypropionitrile | 2141-62-0 | Oxybis(2-ethyl acetate) | |
| 2-Ethylacrylaldehyde oxime | Oxybis(2-ethyl benzoate) | ||
| 2-Ethylbutanol | 97-75-0 | b,b-Oxydipropionitrile | |
| Ethyl-b-ethoxypropionate | 763-69-9 | 1-Pentene | 203-694-5 |
| 2-Ethylhexanal | 123-05-7 | Phenoxyacetyl chloride | 211-862-4 |
| Ethyl vinyl ether | 109-92-2 | 2-Phenoxypropionyl chloride | 122-35-0 |
| Furan | 110-100-9 | Phenyl-o-propyl ether | |
| 2,5-Hexadiyn-1-ol | p-Phenylphenetone | ||
| 4,5-Hexadien-2-yn-1-ol | n-Propyl ether | 111-43-7 | |
| n-Hexyl ether | 112-58-3 | n-Propyl isopropyl ether | |
| Isoamyl benzyl ether | Sodium 8-11-14-eicosatetraenoate | ||
| Isobutyl vinyl ether | 109-53-5 | Sodium ethoxyacetylide | |
| Isophorone | 78-59-1 | Tetrahydropyran | 142-68-7 |
| Isoamyl ether | 544-01-4 | Triethylene glycol dipropionate | |
| b-Isopropoxypropionitrile | 1,3,3-Trimethoxypropane | 241-547-7 | |
| Isopropyl-2,4,5-trichlorophenoxy acetate | 1,1,2,3-Tetrachloro-1,3-butadiene | 921-09-5 | |
| 4-Vinyl cyclohexene | 100-40-3 | ||
| Vinylene carbonate | |||
Source: Kelly, R.J., Review of Safety Guidelines for Peroxidizable Organic Chemicals, Chemical Health and Safety, September/October, 1996.
Principle Investigator:________________
Location: __________________________
Date: ______________________________
| Program Administration | Y | N |
|---|---|---|
| Do all research laboratories have a Laboratory Safety Plan? | ||
| Is the plan complete and up to date? | ||
| Does each laboratory have a designated Chemical Hygiene Officer? | ||
| Is the CHO familiar with his/her duties? | ||
| Identification of Hazards | Y | N |
| Major laboratory hazards are identified and addressed in the Laboratory Safety Plan? | ||
| Laboratory personnel know how to get chemical safety information? | ||
| Chemical safety information is included in the laboratory safety plan? | ||
| Incompatible chemicals are separated? | ||
| Are labels left on incoming containers? | ||
| Is Picric Acid labeled with date of purchase, date of expiration, and is it maintained wet? | ||
| Are Peroxide forming chemicals labeled with date of purchase, date of expiration? | ||
| Are secondary containers labeled in accordance with the laboratory safety plan? | ||
| Are compressed gases secure? | ||
| Is the composition of laboratory-generated mixtures attached to the Laboratory Safety Plan? | ||
| Is the chemical inventory accurate? | ||
| Is a spill kit available? | ||
| Prior Approval Procedures | Y | N |
| Are there any operations or activities that require prior approval before performing? | ||
| Are these documented in the Laboratory Safety Plan? | ||
| Carcinogens, genotoxins, reproductive toxins, and compounds with a high degree of acute toxicity | Y | N |
| Is there an adequate procedure for identifying carcinogens, genotoxins, reproductive toxins, and compounds with a high degree of acute toxicity used in the laboratory? | ||
| Have they been identified in this laboratory? | ||
| Are there written SOPs for handling carcinogens, genotoxins, reproductive toxins, and compounds with a high degree of acute toxicity? | ||
| Are they followed? | ||
| Are areas or hoods where these substances are in use posted with a designated area sign? | ||
| Engineering Controls | Y | N |
| Are laboratory hoods and local exhaust systems provided where needed? | ||
| Are hoods inspected annually? | ||
| Is there an inspection sticker? | ||
| Personal Protective Equipment | Y | N |
| Has the correct PPE been selected based on a document analysis of the hazards? | ||
| Is PPE available as needed? | ||
| Have workers been trained on when and how to use their PPE? | ||
| Do laboratory workers use required PPE? | ||
| Training | Y | N |
| Have all laboratory workers received Chemical Hygiene Plan training? | ||
| Have all laboratory workers received training on the Laboratory Safety Plan and all hazards present in the laboratory? | ||
| Is the training documented? | ||
| Exposure Monitoring | Y | N |
| Have any potential exposures above the allowable limit (Action Level or Permissible Exposure Limit) been identified? | ||
| Has the Ames Chemical Hygiene Officer been contacted to schedule air-sampling? |
Example questions which laboratory personnel should be able to answer:
![]()