
Ames Procedural
Requirements
APR 8715.1
Effective Date: 09/10/2012
Expiration Date: 09/10/2017
COMPLIANCE IS MANDATORY
Ames Health and Safety Procedural Requirements
32.1.1.1 Monitor the Ames Bloodborne Pathogen Program to include assessments of work-sites and employee procedures where there is a reasonable anticipated potential of exposure to blood or OPIM.
32.1.1.2 Advise Ames management on matters concerning bloodborne pathogens.
32.1.1.3 Investigate exposure incidents and report findings to Ames management and agencies as required.
32.1.1.4 Review and update the Exposure Control Plan annually and whenever necessary to reflect new or modified tasks and procedures, which affect occupational exposure.
32.1.1.5 Solicit input from non-managerial employees responsible for direct patient care who are potentially exposed to injuries from contaminated sharps in the identification, evaluation, and selection of effective engineering and work practice controls and -document the solicitation in the Exposure Control Plan.
32.1.1.6 Review periodically with revision and update if needed. Revisions will incorporate:
32.1.2.1 Identify any procedures, positions, and new or revised tasks that involve occupational exposure to bloodborne pathogens, and communicate program plans and changes relevant to that exposure to the Safety Division.
32.1.2.2 Ensure that employees with occupational exposure shall receive timely orientation and training (see section 32.3) using the New Employee Orientation Checklist provided in Appendix C.
32.1.2.3 Ensure that, as soon as a new employee is hired for tasks with occupational exposure, the supervisor enrolls the employee in Bloodborne Pathogens basic training and schedules Hepatitis B vaccination consultation at the Ames Health Unit (by submittal of the form in Appendix D, (section 32.10.2). Within ten working days of the initial assignment, the Hepatitis B vaccination is made available to the employee after BBP training. General and task-specific safety training is also scheduled or provided by the supervisor. A New Employee Orientation Checklist for supervisors is provided in Appendix C.
32.1.2.4 Complete the Ames Safety Accountability Program (ASAP) medical survey and refer to the Ames Health Unit for Hepatitis B consultation and vaccination, using the form and checklist provided in Appendix I.
Note: Associates and students who will perform tasks under this program shall complete medical evaluation and vaccination at their institution and provide a copy of their health care professional's written clearance to their supervisor at the start of their assignment.
32.1.2.5 Perform and coordinate required responses to exposure incidents, using forms and checklist provided at the end of this document:
32.1.2.6 Maintain documentation for specific task controls, cleaning schedules and records, inspection schedules and records, and operating procedures, as appropriate. (Sample forms are available at the end of this document for optional use.)
32.1.2.7 Identify persons who may be expected to provide first aid or Cardio Pulmonary Resuscitation (CPR) in an occupational emergency situation as designated emergency responders (for example, Disaster Assistance Response Team (DART) members). Include an appropriate job element in their performance plan, and ensure that designated responders receive regular annual and supplemental training as required in the Exposure Control Plan (section 32.2).
Note: There shall be no undocumented or informal expectation that any employee will provide first aid and/or CPR.
32.1.3.1 Before working with human blood or other potentially infectious materials employees shall;
32.1.3.1.1 Attend Bloodborne Pathogen training
32.1.3.1.2 Complete the required medical consultation
32.1.3.1.3 Use universal precautions
32.1.3.1.4 Follow engineering and work practice controls (as defined in this chapter).
32.1.3.1.5 Report mishaps
32.1.4.1 Provide medical consultation and offer Hepatitis B vaccination series to NASA employees who anticipate occupational exposure.
32.1.4.2 Retain medical records for each NASA employee with occupational exposure in accordance with 29 CFR 1910.1020. Records will be retained for 30 years post employment. HIV test requests are maintained as separate coded records
32.1.4.3 Medical records are confidential and will not be disclosed or reported without the employee's express written consent to any person within or outside the workplace, except as may be required by law. These records are provided for examination and copying to the subject employee upon request. Any records generated for a non-NASA employee will be released to a health care provider designated by the employee.
Note: In the event of any bloodborne pathogen exposure incident, the Office of the Chief Counsel shall be called for consultation and advice regarding content, disclosure, and release of medical records.
32.1.4.4 Maintain Ames medical records as specified in 29 CFR 1910.1030..
32.1.4.5 Provide emergency care to any person who experiences exposure while providing emergency aid as a Good Samaritan act at Ames, and, with consent, transfers relevant medical records to the health care provider designated by the exposed person.
32.1.4.2.1Pre-exposure Evaluation and Vaccination
32.1.4.2.2 Hepatitis B Vaccination, Post-Exposure Evaluation and Follow-Up
32.1.5.1 Ensure that the onsite manager is knowledgeable regarding OSHA Bloodborne Pathogens regulations, provide resources, and direction for compliance with all requirements.
32.1.5.2 Review and evaluate performance of the contractor's Exposure Control Plan.
32.1.5.3 Verify contractor's written Exposure Control Plan and procedures are in compliance with all elements of the standards.
32.1.6 identify positions or operations that are subject to Bloodborne Pathogens regulations. Contractors who perform tasks with occupational exposure shall be covered by their employer's Bloodborne Pathogens Protection Program with exposure controls that comply, at a minimum, with the provisions of this Plan.
32.2.1.1 Civil Service Job Classifications with Possible Bloodborne Pathogen Exposure
There are no civil service job classifications at Ames with all persons having reasonably anticipated exposure to bloodborne pathogens.
Civil service job classifications in which some Ames personnel may have reasonably anticipated exposure include:
| Occupation Code | Position Title | Task with Potential BBP Exposure |
|---|---|---|
| 80 | Physical Security Specialist | Emergency Response |
| 80 | Security Officer | |
| 80 | Security Specialist | |
| 401 | AST, Neurobiological Studies | Handling of human tissue, organs or OPIM |
| 404 | Biological Laboratory Technician | |
| 413 | Physiologist | |
| 499 | Student Trainee, Biological Science | |
| 601 | Human Research Manager | |
| 602 | Medical Officer | Patient Care |
| 690 | Industrial Hygienist | Emergency Response |
| 1301 | AST, Life Support Studies | Handling of human tissue, organs or OPIM |
| 4952 | Environmental Compliance Specialist | Monitoring Sanitary Waste System |
All persons whose position description includes the following job classifications have reasonably anticipated exposure. These are typically contractor positions. Positions with bloodborne pathogen exposure:
| Position Title | Task with Potential BBP Exposure |
|---|---|
| Physician | Patient Care |
| Nurse | |
| Phlebotomist | |
| Security officer | Emergency Response whose position description includes the following job classifications have reasonably anticipated exposure, when assigned listed tasks. |
| Law enforcement officer | |
| Security shift supervisor | |
| Designated emergency responder | |
| Technician (laboratory science) | Handle human tissue products, e.g., human cell culture, standard for blood analyzer, and human serum albumin. |
| Research scientist | |
| Research associate | |
| Payload scientist | |
| Experiment support scientist | |
| Plumber | Clean sewer (when expected to contain sharps and/or contaminated material) |
The following job classifications have specific prohibited activities:
| Position | Prohibited activities, listed for contingency operations only |
|---|---|
| Custodian/janitor | Pick up waste containing sharps |
| Clean undisinfected area following injury or illness Clean Occupational Health Unit contaminated area Clean clinical research laboratory contaminated area Clean and/or autoclave contaminated labware |
|
| Hazardous waste technician | Handle improperly packaged biohazardous waste |
| Incinerator operator | Handle improperly packaged biohazardous waste |
This section contains mandatory compliance methods specified in 29 CFR 1910.1030. Managers or supervisors shall develop site-specific guidelines for conducting tasks with BBP exposure. A worksheet is provided in Appendix H for documentation of the controls and PPE provided for tasks with anticipated exposure. The worksheet is attached to the local (e.g., organization, site, or project) safety plan, with any standard operating procedures (SOPs) and other appropriate supplemental documentation.
Universal Precautions are adopted as the primary rule to prevent exposure to potentially infectious materials. All body fluids shall be considered potentially infectious. Universal Precautions, are standard practice for all procedures in which exposure to body fluids or OPIM is possible.
32.2.5.1 General controls specified by the Bloodborne Pathogens Standard are as follows:
32.2.5.2 Eating, drinking, smoking, applying cosmetics or lip balm, and handling contact lenses are prohibited in work areas where there is a reasonable likelihood of occupational exposure.
32.2.5.3 Food and drink will not be kept in refrigerators, freezers, shelves, cabinets, or on countertops where blood or other potentially infectious materials may be or may have been used or stored.
32.2.5.4 Specimens of blood or other potentially infectious materials will be placed in containers that prevent leakage during collection, handling, processing, storage, transport, or shipping.
32.2.5.5 All containers will be identified by the Biohazard symbol and legend (predominantly fluorescent orange or orange-red with lettering and symbols in a contrasting color) and a label identifying the date, contents, and responsible person. Containers must be:
32.2.5.6 Biohazard warning labels will be affixed to refrigerators and freezers that contain blood or other potentially infectious materials (an example is provided in Appendix M.)
32.2.5.7 All procedures that involve blood or other potentially infectious materials shall be performed in a manner that minimizes splashing, spraying, spattering, and generating droplets of these substances. If possible, blood samples will be capped during centrifugation. Operations that generate aerosols will be conducted within an exhaust or biohazard containment hood or glove box.
32.2.5.8 Pipettes, suction tubes, or other equipment may not be placed in the mouth while in an area where blood or other potentially infectious materials are present.
32.2.5.9 Devices that offer an alternative to needles (stopcocks, needle-protected systems, or needleless systems) or self-sheathing needles shall be used whenever possible.
32.2.5.10 When it is not possible to use self-sheathing needle syringes and the employee must recap, some type of device that protects the hand or allows a safe one-handed recapping method must be used.
32.2.5.11 A proper, one-handed scoop method is a work practice that may also be used in these circumstances.
32.2.5.12 A written justification for recapping shall be included in the worksite exposure control plan.
32.2.5.13 Shearing or breaking of contaminated needles is prohibited. Contaminated needles and other contaminated sharps shall not be bent, recapped, or removed by hand as general practice. In circumstances where these actions are necessary, a written justification is included in the worksite exposure control plan. Such actions may be accomplished only through the use of a mechanical device or a one-handed technique.
32.2.5.14 Contaminated disposable sharps are placed in red Sharps Containers (immediately or as soon as possible after use). Disposable sharps containers shall be:
32.2.6.1 Personal Protective Equipment (PPE) is selected for each task with occupational exposure. PPE provides an effective barrier to the passage of potentially infectious materials to or through the employees' clothing or prevents contact with skin, eyes, mouth, or other mucous membranes, for the duration of use. Selection of PPE is performance based. A supply of the designated PPE (in the appropriate size, where applicable, for each involved employee) is maintained in each workplace where potentially infectious materials may be present. Any employee who declines to use the provided PPE for any reason shall confer with the supervisor and/or the Safety Office in order to determine alternative protection. Appropriate PPE is available from Ames Stores Stock.
32.2.6.1.1 Gloves must be worn when it is reasonably anticipated that the employee may have hand contact with blood, other potentially infectious materials, mucous membranes, or non-intact skin.
32.2.6.1.2 Gloves must also be worn when performing vascular access procedures, and when handling or touching contaminated items or surfaces.
32.2.6.1.3 Disposable (single use) gloves such as surgical or examination gloves are replaced as soon as possible when contaminated or as soon as feasible if they are torn, punctured, or when their ability to function as a barrier is compromised. Disposable gloves shall not be washed or decontaminated for reuse.
32.2.6.1.4 Masks, in combination with eye protection devices, such as goggles or glasses with solid side shields or chin-length face shields, shall be worn whenever splashes, spray, spatter, or droplets of blood or other potentially infectious materials may be generated and eye, nose, or mouth contamination can be anticipated.
32.2.6.1.5 Appropriate protective clothing such as, but not limited to, gowns, aprons, lab coats, clinic jackets, or similar outer garments must be worn in occupational exposure situations. The type and characteristics depend upon the task and degree of exposure anticipated.
32.2.6.1.6 All PPE will be removed prior to leaving the work area.
32.2.6.1.7 When PPE is removed it shall be placed in an appropriately designated area or biohazard container for storage, washing, decontamination, or disposal.
32.2.6.1.8 Soiled laundry shall be handled, stored, and processed in a manner that prevents the spread of infection and assures the maintenance of clean linen.
32.2.6.1.9 Soiled laundry shall be stored and handled separately from clean linen.
32.2.6.1.10 Personnel handling soiled laundry will use gloves and wash hands after handling.
32.2.6.1.11 Carts used to transport soiled laundry shall be so labeled, cleaned daily, and not used for clean linen.
32.2.6.1.12 Contaminated laundry shall not be sorted or rinsed, and will be contained with a minimum of agitation in closed, labeled containers or red bags at the location where it was used.
32.2.6.1.13 Wet items will be double bagged.
32.2.6.1.14 Resuscitator devices shall be readily available and accessible to persons designated as emergency care providers (e.g., security guard, DART member).
32.2.6.1.15 These shall include emergency ventilation devices such as masks, mouthpieces, resuscitation bags, and shields/overlay barriers.
32.2.7.1 Supervisors shall ensure that the worksite is maintained in a clean and sanitary condition. The supervisor establishes and posts an appropriate written schedule for cleaning of equipment and environmental and working surfaces.
32.2.7.2 A sample BBP Incident Inspection Supplemental Checklist (Appendix J) shall be used as a supplement to the general laboratory inspection checklist.
32.2.7.2.1 Contaminated work surfaces shall be decontaminated with an appropriate EPA-approved disinfectant after completion of procedures; immediately or as soon as possible when surfaces are overtly contaminated or after any spill of blood or other potentially infectious materials; and at the end of the work shift if the surface may have become contaminated since the last cleaning.
32.2.7.2.2 Protective coverings, such as plastic wrap, aluminum foil, or imperviously backed absorbent paper used to cover equipment and environmental surfaces, are removed and replaced as soon as possible when they become overtly contaminated or at the end of the work shift if they are contaminated during the shift.
32.2.7.2.3 All bins, pails, cans, and similar receptacles intended for reuse that may become contaminated with blood or other potentially infectious materials are inspected and decontaminated on a regularly scheduled basis, and cleaned and decontaminated immediately or as soon as possible when overtly contaminated.
32.2.7.2.4 Broken glassware that may be contaminated shall not be picked up directly with the hands. It is cleaned up using mechanical means, such as a brush and dustpan, tongs, or forceps.
32.2.7.2.5 Laboratory spills are cleaned up by the staff, following the decontamination guidelines provided in Appendix L. In the event of an unusual or particularly large spill, involved personnel shall contact the Safety Division for assistance.
32.2.7.2.6 In the event of a spill of infectious material in a public access area (e.g., hallway, elevator), involved personnel shall keep all persons away from the spill area and call the Dispatch Office at 4-5416.
32.2.7.2.7 Disinfection of spills that involve blood or OPIM is the responsibility of the involved staff or emergency responders. The disinfection process must be completed before the custodial staff is contacted for cleanup.
32.2.7.2.8 The custodial staff will not clean up spills prior to their disinfection.
32.2.7.2.9 Contaminated laundry is:
32.2.8.1 This chapter, which contains the Ames Bloodborne Pathogens Exposure Control Plan, shalll be provided to each employee with occupational exposure to bloodborne pathogens at the time of initial Bloodborne Pathogens Standard training.
32.2.8.2 Copies shall be made available to each employee at annual refresher training.
32.2.9.1 The Exposure Control Plan is accessible to employees by contacting the Safety, Health and Medical Division (4-5602)
32.2.10.1 Warning labels will be affixed to containers of regulated waste, refrigerators, and freezers that contain blood or other potentially infectious material. Warning labels are also affixed to other containers used to store, transport, or ship blood or other potentially infectious materials. Individual containers that are placed in a labeled container during storage, transport, shipment, or disposal do not require warning labels. However, any such container that has the potential to become separated from its labeled outer container shall be labeled.
32.2.10.2 Labels will be either an integral part of the container, or will be affixed as close as possible to the container by string, wire, adhesive, or other method that prevents the loss or unintentional removal of the label.
32.2.10.3 Labels required by the Bloodborne Pathogens standard shall be predominantly fluorescent orange or orange-red with lettering and symbols in a contrasting color, except for labels on red bags or red containers, which do not need to be color-coded. Labels include the biohazard symbol with either BIOHAZARD or, in the case of regulated waste, BIOHAZARDOUS WASTE.

NOTE: Guidelines for labels required for materials and waste at Ames are provided in Appendix M, and also in the Ames Environmental Management Handbook.
32.3.1.1 Employees with occupational exposure shall participate in a training program that is provided during working hours and at no cost. The training program is given as followings:
32.3.1.1.1. Within ten (10) days of initial assignment.
32.3.1.1.2. At least annually (after initial training).
32.3.1.1.3 When new or modified tasks or procedures affect occupational exposure.
32.3.2.1 Training is made available to government employees by the Safety Health, and Medical Services Division (Code QH). Bloodborne Pathogens Training (BBPT) provides instruction in the elements specified in 29 CFR 1910.1030, which include:
32.3.2.1.1. An accessible copy of the regulatory text of the Bloodborne Pathogens Standard and an explanation of its contents.
32.3.2.1.2. A general explanation of the epidemiology and symptoms of bloodborne diseases.
32.3.2.1.3. An explanation of the modes of transmission of bloodborne pathogens.
32.3.2.1.4. An explanation of the employer's exposure control plan and the means by which the employee can obtain a copy of the written plan.
32.3.2.1.5. An explanation of the appropriate methods for recognizing tasks and other activities that may involve exposure to blood and other potentially infectious materials.
32.3.2.1.6 An explanation of the use and limitations of methods that will prevent or reduce exposure including appropriate engineering controls, work practices, and Personal Protective Equipment (PPE).
32.3.2.1.7 Information on the types, proper use, location, removal, handling, decontamination and disposal of Personal Protective Equipment (PPE).
32.3.2.1.8 An explanation of the basis for selection of Personal Protective Equipment (PPE).
32.3.2.1.9 Information on the Hepatitis B vaccine, including information on its efficacy, safety, method of administration, the benefits of being vaccinated, and that the vaccine and vaccination is offered free of charge.
32.3.2.1.10 Information on the appropriate actions to take and persons to contact in an emergency that involves blood or other potentially infectious materials.
32.3.2.1.11 An explanation of the procedure to follow if an exposure incident occurs, including the method of reporting the incident and the medical follow-up that will be made available.
32.3.2.1.12 Information on the post-exposure evaluation and follow-up that the employer is required to provide for the employee following an exposure incident.
32.3.2.1.13 An explanation of the required signs and labels and/or color-coding.
32.3.2.1.14 An opportunity for interactive questions and answers with the person conducting the training session.
32.3.3.1 Training records shall be retained by the Safety, Health and Medical Services Division (Code QH) for each employee with occupational exposure.
32.3.3.2 Records shall be retained for a minimum of three years from the date on which the training occurred.
32.3.3.3.The records must be available upon request to the employee and his/her supervisor, and to employee representatives, Ames management, and regulatory agencies.
32.3.3.4 Training records must include, at a minimum:
32.4.1 The first priorities in response to any mishap that involves personnel are to obtain emergency assistance, if needed, and to prevent further injury or damage. Actions to be taken by the exposed employee, the cognizant supervisor, and Ames Health Unit are described below.
32.4.2 Any incident with possible bloodborne pathogen exposure must be reported to the Ames supervisor, who must ensure that response actions are performed.
32.4.1.1.1 In the event of any known or suspected occupational exposure to blood or potentially infectious materials, the exposed employee must report promptly to the Ames Health Unit for medical aid and consultation. After hours, the employee will call 911 (or the Ames Dispatch Office) for assistance in reporting to a local hospital emergency room.
32.4.1.1.2 The employee or involved coworker must promptly report the mishap to his/her supervisor. The scene may be preserved, if appropriate, for accident investigation, while preventing subsequent exposure risk.
32.4.1.1.3 It is the employee's responsibility to coordinate scheduling of appointments for treatment, testing, evaluation, discussion, and counseling with his/her supervisor, and to request transportation (to be provided by Ames) if needed.
32.4.1.2.1 The cognizant supervisor is responsible for ensuring that the exposed employee receives all appropriate medical care, for investigating and reporting the incident, and for immediate and ultimate corrective actions. If any unaltered source material remains (for example, blood in a test tube), the supervisor shall seal, label, and deliver it to the Ames Health Unit for possible testing. If the source individual is known or can be identified, it is appropriate for the supervisor to suggest that the individual report to the Ames Health Unit for consultation, without discussion of blood testing.
32.4.1.2.2 These proceedings should be documented; however, the source individual may not be named in the record without written consent. It is the supervisor's responsibility to enable the employee to schedule and attend convenient appointments for consultation, treatment, and counseling.
32.4.1.2.3 If the Ames Health Unit does not provide exposure incident services, the supervisor shall coordinate arrangements for care and for transfer of documentation to the health care provider, as well as ensure that medical records are transferred from the health care professional to the Ames Occupational Health Unit for the employee's file.
32.4.1.2.4 An Exposure Incident Action Checklist for Supervisors is provided in Appendix E to facilitate documentation of required actions.
32.4.1.2.5 Supervisors must refer to APR 1700.1 Chapter 4, Mishap Reporting and Investigating. To report a mishap or close call incident notify the Ames Safety Office and file a written report, with as much information as possible. Use the Incident Reporting Information System (IRIS)
32.4.1.2.6 The initial report must be forwarded to the Safety Division within 24 hours of the incident.
32.4.1.2.7 Bloodborne Pathogens Standards specify information that must be included in the mishap description. The Exposure Incident Description form is provided in Appendix F to facilitate inclusion of all required information in the report.
32.4.1.2.8 The exposed employee's supervisor shall provide this information (as completely as possible) to the Ames Health Unit (or other health care provider) when the employee is treated and evaluated. It may be appropriate to provide a more complete incident description in the final report. However, confidential information, including medical diagnosis and test results, may not be revealed. Supervisors are advised to consult with the Office of the Chief Counsel before submission of the final mishap report.
32.4.1.2.9 The final NASA Mishap Report (NASA 1627) MUST contain an evaluation of policies, engineering controls, and work practices in place, and failures of control at the time of the incident, including discussion of the effectiveness of protective equipment and clothing used.
32.4.1.2.10 It must also contain documentation of actions taken and a schedule (with dates) for actions planned to prevent recurrence. Appendix E, is provided for this purpose.
32.4 2.1 Following a report of an exposure incident, the Ames Health Unit provides a confidential medical evaluation and follow-up to the exposed employee, in accordance with Center for Disease Control guidelines. This follow-up includes obtaining blood specimens from the employee and from the source individual, where feasible, when consent is obtained, and the maintenance of required medical records.
32.4.2.2 Medical counseling is provided by Ames Health Unit health care professionals. Confidential psychological counseling is available to Government employees, at no cost, through the Employee Assistance Program. Information about this counseling program is available from the Safety Division.
32.4.2.3 Some responsibilities of the employer are delegated to the Ames Health Unit, acting as provider of emergency and follow-up health care, and as the maintainer of Ames medical records. The Ames Health Unit, as the Governments representative, shall, with consent, provide relevant records to the health care provider for any exposed individual, subject to consultation and advice by Office of the Chief Counsel regarding legal restrictions.
32.4.2.4 When a Government employee presents for treatment and evaluation of possible bloodborne pathogens exposure, the Ames Health Unit shall determine if exposure has occurred and initiate appropriate actions.
32.4.2.5 These may include evaluation, consultation, testing, and prophylaxis, if indicated, for the exposure and any reported illnesses, and shall provide a health care professional's written opinion to the exposed employee within 15 days of evaluation.
32.4.2.6 The written opinion will meet all of the requirements as listed in 29 CFR 1910.1030 (f). All other findings or diagnosis shall remain confidential and shall not be included in the written report.
32.4.2.7 An exposed employee shall be informed of the results of the source material or, with consent, the results of the source individual's testing for Hepatitis B Virus and Human Immunodeficiency Virus, and told about any medical conditions resulting from the exposure that may require further evaluation or treatment, while still maintaining confidentiality as required by law. California Health and Safety Code Sec. 199.21 specifically prohibits the disclosure of results of an HIV test in a manner that identifies or provides identifying characteristics of the person to whom the test results apply, except pursuant to written authorization, or when another statute expressly provides an exemption. Civil penalties can be assessed against violators of this statute. Such test results can be revealed, however, with the express, written consent of the source individual.
32.4.2.8 The Ames Health Unit provides emergency services, including offer of HBV vaccination, to any person who experiences an exposure incident at Ames in performance of duties or as a provider of emergency first aid, whether as collateral duty or as a Good Samaritan.
32.4.2.9 This chapter, which contains the Ames Bloodborne Pathogens Exposure Control Plan, must be provided to each employee with occupational exposure at the time of initial Bloodborne Pathogens Standard training.
32.4.2.10 Copies shall be made available to each employee at annual refresher training.
A1. Biological Cabinet: A device enclosed except for necessary exhaust purposes on three sides and top and bottom, designed to draw air inward by means of mechanical ventilation, operated with insertion of only the hands and arms of the user, and in which virulent pathogens are used. Biological cabinets are classified as:
A1.1 Class I: A ventilated cabinet for personnel protection with an unrecirculated inward airflow away from the operator and high-efficiency particulate air (HEPA)-filtered exhaust air for environmental protection.
A1.2 Class II: A ventilated cabinet for personnel, product, and environmental protection having an open front with inward airflow for personnel protection, HEPA-filtered laminar airflow for product protection, and HEPA-filtered exhaust air for environmental protection.
A1.3 Class III: A totally enclosed, ventilated cabinet of gas-tight construction. Operations in the cabinet are conducted through attached protective gloves.
A2. Blood: Human blood, human blood components, and products made from human blood.
A3. Bloodborne Pathogens (BBP): Pathogenic microorganisms that are present in human blood and can cause disease in humans. These pathogens include hepatitis B virus (HBV), hepatitis C virus (HCV), human immunodeficiency virus (HIV), and any pathogenic microorganism that is present in human blood and can infect and cause disease in persons who are exposed to blood containing the pathogen.
A4. Clinical Laboratory: A workplace where diagnostic or other screening procedures are performed on blood or other potentially infectious materials.
A5. Contaminated: The presence or the reasonably anticipated presence of blood or other potentially infectious materials on a surface or in an item.
A6. Contaminated laundry: Laundry that has been soiled with blood or other potentially infectious materials.
A7. Contaminated sharps: Any contaminated object that can penetrate the skin.
A8. Decontamination: The use of physical or chemical means to remove, inactivate, or destroy bloodborne pathogens on a surface or item to the point where they are no longer capable of transmitting infectious particles and the surface or item is rendered safe for handling, use, or disposal.
A10. Engineering controls: Controls (e.g., sharps disposal containers, self-sheathing needles) that isolate or remove the bloodborne pathogens hazard from the workplace.
A11. Exposure Incident: A specific eye, mouth, other mucous membrane, non-intact skin, or parenteral contact with blood or other potentially infectious materials that results from the performance of an employee's duties.
A12. Handwashing facilities: A facility that provides an adequate supply of running potable water, soap, and single-use towels or hot air-drying machines.
A13. Licensed health care professional: A person whose legally permitted scope of practice allows him/her to independently perform the activities required by 8 CCR 5193 subsection (f) and 29 CFR 1910.1030 subsection (f), Hepatitis B Vaccination and Post-exposure Evaluation and Follow-Up.
A14. Occupational exposure: Reasonably anticipated potential for exposure to blood or other potentially infectious materials that may result from the performance of an employee's duties.
A16. One-hand Technique: Procedure wherein the needle of a reusable syringe is capped in a sterile manner during use. The technique employed shall require the use of only the hand holding the syringe so that the free hand is not exposed to the uncapped needle.
A17. Other potentially infectious materials (OPIM):
A17.1 The following human body fluids: semen, vaginal secretions, cerebrospinal fluid, synovial fluid, pleural fluid, pericardial fluid, peritoneal fluid, amniotic fluid, saliva in dental procedures, any other body fluid that is visibly contaminated with blood such as saliva or vomitus, and all body fluids in situations where it is difficult or impossible to differentiate between body fluids such as emergency response;
A17.2 Any unfixed tissue or organ (other than intact skin) from a human (living or dead); and
A17.3 HIV-containing cell or tissue cultures, organ cultures, and HIV- or HBV-containing culture medium or other solutions; and blood, organs, or other tissues from experimental animals infected with HIV or HBV. Preparations that originate from body fluids, such as human serum albumin, shall also be considered potentially infectious materials, unless specifically excluded or certified pathogen-free. Coverage under this definition also extends to blood and tissues of animals that are deliberately infected with HIV or HBV.
A18. Parenteral: Piercing mucous membranes or the skin barrier through such events as needlesticks, human bites, cuts, and abrasions.
A19. Personal Protective Equipment (PPE): Specialized clothing or equipment worn or used by an employee for protection against a hazard. General work clothes (for example, uniforms, pants, shirts, or blouses) not intended to function as protection against a hazard are not considered to be personal protective equipment.
A20. Regulated waste: Liquid or semiliquid blood or other potentially infectious materials; contaminated items that would release blood or other potentially infectious materials in a liquid or semiliquid state if compressed; items that are caked with dried and are capable of releasing these materials during handling; contaminated sharps; and pathological and microbiological wastes containing blood or other potentially infectious materials. Regulated Waste includes "medical waste" regulated by California Health and Safety Code 117600; Medical Waste Management Act.
A21. Research laboratory: A laboratory that produces or uses research-laboratory-scale amounts of HIV or HBV. Academic research laboratories are included in this definition. Laboratories that conduct research unrelated to HIV or HBV on blood and other body fluids, or that use unconcentrated blood or blood components as the source of HIV or HBV, are not considered research laboratories for the purpose of the standard.
A22. Sharps: Needles, scalpels, and any other object that may produce a puncture wound that would expose employees to blood or OPIM (for example, wires, broken glass).
A23. Source individual: Any individual, living or dead, whose blood or other potentially infectious materials may be a source of occupational exposure to the employee.
A24. Sterilize: The use of a physical or chemical procedure to destroy all microbial life, including highly resistant bacterial endospores. Sterilization includes procedures regulated by California Health and Safety Code Section 118215.
A25. Universal Precautions: An approach to infection control in which all human blood, and human body fluids are treated as if known to be infectious. Universal precautions do not apply to feces, nasal secretions, sputum, saliva, sweat, tears, urine, and vomitus, unless they contain visible blood.
A26. Work practice controls: Controls that reduce the likelihood of exposure by altering the manner in which a task is performed (e.g., prohibiting recapping of needles by a two-handed technique).

TO: Ames Health Unit DATE: FROM: NAME (Supervisor): Org: Mail Stop: Phone: Employee Name: Mail Stop: Phone: Bloodborne Pathogens Assignment Start Date:
The above named employee has been assigned tasks with potential bloodborne pathogens exposure. Please establish a medical record for this employee, and make Hepatitis B vaccination available to him/her unless he/she has previously received the complete Hepatitis B vaccination series, antibody testing has revealed that the employee is immune, or the vaccine is contraindicated for medical reasons.
Participation in a prescreening program is not a prerequisite for receiving Hepatitis B vaccination.
An employee who declines to accept Hepatitis B vaccination shall sign the declination statement (DQH-BBP4) to be retained in his/her medical record.
If the employee initially declines Hepatitis B vaccination but at a later date, while still covered by the Standard, decides to accept the vaccination, please provide it for him/her.
If a routine booster dose(s) of Hepatitis B vaccine is recommended by the U.S. Public Health Service at a future date, please provide such booster dose(s) to the employee.
|
Employee Name:__________ Date Of Incident:_____ Date/Initial:______ |
|
|---|---|
| 1. | Secure area, obtain aid if needed |
| 2. | Ensure that either employee goes to Occupational Health Unit or other health care professional is made available to employee |
| 3. | Identify witnesses and involved personnel |
| 4. | Identify source of exposure (individual or material) |
| 5. | Send source individual or material to Occupational Health Unit or other health care provider for testing; if consent cannot be obtained, send documentation to Occupational Health Unit |
| 6. | Determine circumstances, record on Exposure Incident Description (32.10.4) |
| 7. | Take Exposure Incident Description (32.10.4) to Occupational Health Unit If employee goes to other health care professional, ensure that Exposure Incident Description (32.10.4) and other mandatory information (32.10.12) is provided to the health-care professional |
| 8. | Notify the Safety Office of exposure incident |
| 9. | Supervise cleanup and area follow-up, if any |
| 10. | Submit NASA Mishap Report to Safety Office (QH) within 24 hours using IRIS (nasa.ex3host.com/IRIS). |
| 11. | Investigate causes, schedule appropriate actions; record on Exposure Incident Evaluation (32.10.5) |
| 12. | Ensure that the employee receives a copy of the health care professional's written opinion (within 15 days of evaluation) |
| 13. | Contact Ames Legal Office; schedule consultation with employee and other involved persons regarding confidentiality of source name and test results. |
| 14. | If the employee does not use the Health Unit as health care provider, ensure that the employee receives the results of tests on source blood and that copies of test results are forwarded to the Health Unit. |
| 15. | Ensure that the employee is enabled to receive all recommended testing, prophylaxis, and treatment, at no cost and at a convenient time and place. |
| 16. | Ensure that a consultation appointment is made with a health care professional, for the employee, at no cost to the employee and at a convenient time and place, to discuss medical status, including any reported illnesses and recommended treatment. |
| 17. | Ensure that the employee is enabled to receive counseling, at no cost and at a convenient time, and place. |
| 18. | Submit NASA Mishap Report (final) to Safety Office (QH) with Exposure Incident Description (32.10.4) and Exposure Incident Evaluation (32.10.5) attached within two weeks. |
Attach to Initial NASA Mishap Report (NASA 1627) Employee Name: Date of Incident: Time of Incident Location of Incident Job Classification Description of employee's duties during the exposure incident Potentially infectious material (PIM) involved: Type Source (name if available) Route of Exposure _____ Needlestick _____ Piercing of skin with contaminated sharp _____ Splashing/spraying of blood or other PIM _____ Other (describe) Circumstances under which the exposure occurred How the incident was caused (accident, equipment malfunction, etc.) Personal Protective Equipment (PPE) being used Actions taken (first aid, decontamination, cleanup) _____________________________________________________________ Supervisor (Printed Name) (Signature) Date
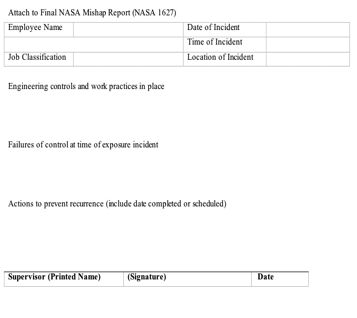
Attach to Final NASA Mishap Report (NASA 1627) Employee Name: Date of Incident Time of Incident Location of Incident Job Classification Engineering controls and work practices in place Failures of control at time of exposure incident Actions to prevent recurrence (include date completed or scheduled) ______________________________________________________________ Supervisor (Printed Name) (Signature) Date
Organization:______________________ Job Classification:_________________ Task Description: Type of BBP exposure risk: Direct contact with blood Direct contact with other fluid (specify) Needle stick Other sharps hazard (specify) Other hazards associated with task: Engineering and Work Practice Controls _ Ventilation _ Housekeeping _ General exhaust _ Cleaning schedule _ Local exhaust hood _ Cleaning/Decontamination methods (specify) _ Tissue culture _ Front door/ceiling filter exhaust to room _ Biohazard containment SUPPLEMENTAL RULES AND PROCEDURES (specify) Personal Protective Equipment (Specify by name, size, and part number) Eye/Face _ Safety glasses _ Goggles _ Face shield Hand _ Gloves, disposable _ Gloves, reusable _ Other Inhalation _ Surgical mask (nuisance protection only) Note: respirators are not authorized for BBP protection and may not be used in BBP worksites except by special arrangement with Code QH. Clothing _ Coat/smock _ Coverall _ Apron _ Gauntlets _ Boots _ Surgical cap _ Other (specify) Comments Completed By: _____________________________________________________________________ Printed Name Signature Date

HEPATITIS B VACCINE
Product Name Date Dose Site Lot# Signature
1.
2.
3.
OCCUPATIONAL EXPOSURE TO HEPATITIS B VIRUS (HBV)
Employee Fact Sheet
HEPATITIS B: Hepatitis B is a viral infection of the liver caused by Hepatitis B virus (HBV). Each year approximately 300,000 new infections are reported to the Center for Disease Control. Most people who become infected with Hepatitis B recover completely, but 5 to 10 percent will become chronic carriers of the virus. Although many chronic carriers do not have symptoms of the disease, they are capable of transmitting the virus to other persons, primarily through blood exposures or sexual contact. Each year 4000 to 5000 persons die from Hepatitis B induced liver disease, cirrhosis, or liver cancer. (Two hundred of these die shortly after initial infection from fulminant HBV).
OCCUPATIONAL EXPOSURE: Health care workers with direct patient contact, laboratory workers and researchers, and other employees with blood or body fluid contact are at increased risk for acquiring the Hepatitis B virus. An unvaccinated individual who receives an accidental blood or body fluid exposure from an infected source has a 40 percent chance of becoming infected with Hepatitis B.
VACCINATION: Becoming infected with Hepatitis B is preventable. The Hepatitis B vaccine, a synthetic vaccine made from a yeast base, is currently being offered to Government employees at risk at no cost to the employee. Full immunization requires completion of a series of three vaccinations given over a six-month period. Eighty to 90 percent of healthy people who receive the vaccine develop antibodies that protect them from getting Hepatitis B. There is no evidence that the vaccine has ever caused Hepatitis B. At this time, no one knows how long the immunity produced by the vaccine will last, and the need for additional vaccinations has not been determined. Health care workers who are immunocompromised or on dialysis might require increased doses of the vaccine in order to convert to positive antibodies. The incidence of side effects is very low. A few persons experience tenderness and redness at the injection site. A low-grade fever may occur. Rash, nausea, joint pain, and mild fatigue have also been reported.
TREATMENT OF EXPOSURE: If the individual has received the Hepatitis B vaccine and has documented antibodies to HBV, no further treatment is necessary at the time of exposure. However, someone who is not protected by the vaccine and does not have antibodies to HBV needs to receive Hepatitis B Immunoglobulin (HBIG) as soon as possible after the exposure. These persons are also encouraged to receive the Hepatitis B vaccine at this time.
If you have any questions about Hepatitis B vaccine, call the Ames Health Unit at 4-5287.

(Source: Draft NIH Exposure Control Plan)
All work surfaces and equipment that come into contact with blood, body fluids, and any infectious agent or materials must be disinfected daily, upon completion of work, with an appropriate disinfectant. Additionally, work surfaces and equipment must be disinfected after any overt spill. Work surfaces should be covered with plastic-backed absorbent toweling to facilitate cleanup and reduce production of aerosols that may result from a spill. Spills within work areas are to be cleaned up by laboratory or research personnel. Housekeeping staff is not authorized to clean up spills of potentially infectious material. Spills of potentially infectious material are to be cleaned up using the following method:
For large spills (over one-half cup), mop using a 1:10 bleach solution (or EPA disinfectant). After cleaning, the mixture in the bucket should be flushed down the toilet.
NOTE: Any contaminated PPE must be bagged for disposal or commercial laundry, and shall not be sorted or rinsed at the location of use. Contaminated PPE may not be taken home by an employee for cleaning.
Note: Contact the Environmental Services Office if a combination of BIOHAZARDOUS WASTE with hazardous chemical waste is anticipated. Waste must be labeled to identify all hazards.
Waste generated from operations with occupational exposure is medical waste when it contains or is visibly contaminated with human blood, fluid, or tissue, or when it contains or is contaminated with pathogenic or infectious microorganisms. Discarded sharps are treated as medical waste, regardless of contamination (except veterinary used sharps). The definition of Medical Waste applies to disposal procedures.
The Bloodborne Pathogens definition of Regulated Waste for purposes of personnel protection differs in that it is a performance-type standard, which bases the distinction between regulated and unregulated waste on the potential to release PIM during handling. Materials that are visibly contaminated with small amounts of blood or OPIM not subject to release as liquid or flakes may not be Regulated Waste but are still medical waste.
Medical waste (solid or liquid) may not be treated (except as inherent in procedures) unless authorized by Environmental Services Division. Authorized procedures are:
Sterilized (autoclaved) waste shall be disposed as ordinary waste, unless it is hazardous chemical waste. Biohazard labels shall be removed or defaced, and red bags placed within opaque bags in order to eliminate any identification of the waste as biohazardous.
Source: American College of Occupational and Environmental Medicine
Sample Exposure Control Plan
Source: American College of Occupational and Environmental Medicine
Sample Exposure Control Plan
No transmission of Hepatitis B virus infection during mouth-to-mouth resuscitation has been documented. However, because of the theoretical risk of salivary transmission of HIVB during mouth-to-mouth resuscitation, special attention should be given to the use of disposable airway equipment or resuscitation bags and the wearing of gloves when in contact with blood or other body fluids. Resuscitation equipment and devices known or suspected to be contaminated with blood or other body fluids should be used once and disposed of or be thoroughly cleaned and disinfected after each use.
Clear plastic facemasks with one-way valves are available for use during mouth-to-mask ventilation. These masks provide diversion of the victim's exhaled gas away from the rescuer and may be used by health-care providers and public safety personnel properly trained in their use during two-person rescue, in place of mouth-to-mouth ventilation. The need for and effectiveness of this adjunct in preventing transmission of an infectious disease during mouth-to-mouth ventilation are unknown. If this type of device is to be used as reassurance to the rescuer that a potential risk might be minimized, the rescuer must be adequately trained in its use, especially with respect to making an adequate seal on the face and maintaining a patent airway. Such a device requires two hands to secure a proper face seal and to maintain an open airway. As an additional precaution, the rescuer may elect to wear latex gloves because saliva or blood on the victim's mouth or face may be transferred to the rescuer's hands.
![]()